Quick Look
Grade Level: 8 (7-9)
Time Required: 45 minutes
Expendable Cost/Group: US $12.50 (initial cost, items can be reused)
Group Size: 1
Activity Dependency:
Subject Areas: Earth and Space
NGSS Performance Expectations:

| MS-PS4-2 |

Summary
Students use the spectrographs from the "Building a Fancy Spectrograph" activity to gather data about light sources. Using their data, they make comparisons between different light sources and make conjectures about the composition of a mystery light source.Engineering Connection
Engineers design spectrographs to use in both ground- and space-based telescopes to help astronomers figure out of what materials stars, planets and planetary atmospheres are made. Designing and developing the spectrographs for space satellites is a special challenge, and requires engineering of lightweight materials as well as durable equipment that can withstand the rigors of space travel.
Learning Objectives
After this activity, students should be able to:
- Describe how patterns can tell us something about what kind of light we see.
- Explain the equipment engineers use to understand atmospheric constituents.
- Understand how spectograph technology is used to explore outer space.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS4-2. Develop and use a model to describe that waves are reflected, absorbed, or transmitted through various materials. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop and use a model to describe phenomena. Alignment agreement: | A sound wave needs a medium through which it is transmitted. Alignment agreement: When light shines on an object, it is reflected, absorbed, or transmitted through the object, depending on the object's material and the frequency (color) of the light.Alignment agreement: The path that light travels can be traced as straight lines, except at surfaces between different transparent materials (e.g., air and water, air and glass) where the light path bends.Alignment agreement: A wave model of light is useful for explaining brightness, color, and the frequency-dependent bending of light at a surface between media.Alignment agreement: However, because light can travel through space, it cannot be a matter wave, like sound or water waves.Alignment agreement: | Structures can be designed to serve particular functions by taking into account properties of different materials, and how materials can be shaped and used. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Develop innovative products and systems that solve problems and extend capabilities based on individual or collective needs and wants.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Colorado - Science
-
Describe methods and equipment used to explore the solar system and beyond
(Grade
8)
More Details
Do you agree with this alignment?
-
Compare and contrast different types of waves
(Grade
8)
More Details
Do you agree with this alignment?
Materials List
Each student needs:
- Spectrograph from the Building a Fancy Spectrograph activity
- Colored pencils
- Copy of Spectral Mystery Worksheet
To share with entire class:
- 1 power supply for gas discharge tubes
- 1 tube of oxygen gas
- 1 tube of nitrogen gas
- 1 tube of air
- 1 additional tube (your choice)
- 1 "mystery tube" that could be either oxygen, nitrogen, or duplicate of the additional tube
- Any additional tubes
Notes on materials:
If resources allow, set up several power supplies as stations around the room.
The (optional) extension activity uses gas discharge tubes. Gas discharge tubes produce an emission spectrum that can be seen when electricity runs through the tube, exciting the atoms in the gas. Although expensive, when handled properly, gas discharge tubes can last for years.
Purchase power supplies and gas discharge tubes from:
Rainbow Symphony Store
https://www.rainbowsymphonystore.com/
Spectrum tube power supply for $199
Nitrogen gas tubes cost $35
Gas tube prices range from $35 to $43
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_spect_activity6] to print or download.Pre-Req Knowledge
Students should have completed the Building a Fancy Spectrograph and Using a Fancy Spectrograph activities.
Introduction/Motivation
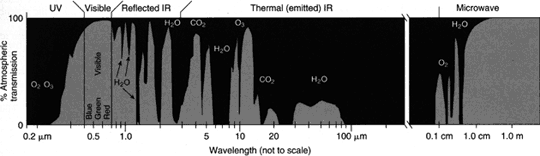
The Earth's atmosphere contains gases that block some kinds of light before they can reach telescopes on the ground. So, to get a complete picture of stellar and planetary spectra, spectrographs are often launched into space, where they do not have to look through the Earth's atmosphere.
Some space spectrographs stay relatively close to Earth, but others, like those aboard NASA's Cassini mission to Saturn, travel into deep space, where they can take an even closer look at the planets.
Getting a spectrograph into space is challenging, because the materials must meet strict weight, strength and temperature requirements. Engineers must design these instruments to have a way to point accurately at a planet or body while collecting data about the object, and have a way to send the information to Earth.
Spectrographs can also be engineered to attach to telescopes on Earth. However, as the telescope is looking through the Earth's atmosphere, it is more difficult to figure out what the atmosphere of a planet contains. When you look through the Earth's atmosphere, dark bands from the Earth's atmosphere appear in your spectrum, creating spectrographic "blind spots" in which scientists cannot learn anything about the objects they are studying.
How do scientists and engineers know what is inside of an atmosphere at all? When astronomers look at the atmosphere of a planet or body in our solar system or beyond, they analyze the spectrum of the atmosphere and match the spectral lines with the lines of known chemicals and elements.
Using your spectrographs today, we are going to look at a few different light sources. We will gather information about these light sources and figure out what is inside of an atmosphere.
Procedure
Terminology Note
In creating the Spectroscopy curricular unit, the Project SPECTRA! program chose to use the term “spectrograph” (as opposed to spectroscope) for the engineering projects activities because a spectrograph is a tool used in spacecraft and modern telescopes, and Project SPECTRA! is an astronomy program. A spectrograph uses a detector, usually a CCD (a charge coupled device, similar to those used in digital cameras), to record the properties of light. Technically, in this unit, students build “spectroscopes,“ which are similar to spectrographs, however, instead of using a detector, the human eye directly observes the light within the scope or projected onto a screen. The primary difference between the two instruments is the method in which the light is detected. A spectrograph enables a person to observe light that cannot be seen with the eye (typically ultraviolet, infrared, and x-rays) because the detector records these wavelengths electronically, enabling the signals to be observed as plots or graphs. In this curricula, when students build their “space-worthy” spectrographs, we consider the students themselves to be the detectors, and leave to their instructors the option of providing students with more in-depth explanation.
Background
See the Procedure-Background section of the Building a Fancy Spectrograph and the Graphing the Rainbow activities.
Light from the Sun travels through the Earth's atmosphere. On its trip through the Earth's atmosphere, the light encounters many atoms and molecules. When light encounters an atom or molecule, it is sometimes absorbed, creating an absorption spectrum. When looking up through the Earth's atmosphere through a spectrograph, lines from the Earth's atmosphere appear in the spectrum. Sometimes the lines are so big and broad; all of the light coming through the atmosphere is absorbed, making it impossible to view the spectra of anything outside of Earth's atmosphere at that wavelength. For example, we cannot see much of anything through the atmosphere in the ultra violet region of the spectrum because the ozone in our atmosphere absorbs most of it, a very good thing for us! Regions in which there are few atmospheric absorption lines in Earth's spectrum are called "transmission windows." The visible wavelength range is one transmission window. Even in a transmission window, it is difficult to view the spectra of other objects because some lines from the Earth's atmosphere are still present. These lines must be removed before we can tell what gasses are from the object and which are constituents of Earth's atmosphere. They are typically removed using computer modeling.
Understanding what the Earth's atmosphere contains is crucial to creating an accurate computer model. Usually, a reference spectrum is created in a laboratory in a similar way to this activity; a spectrograph is used to analyze the air inside a tube of gas at different wavelengths. It is possible to accurately determine the components of Earth's atmosphere in this way.
When light passes through a material (including gases and liquids), many things may happen to it:
Absoprtion 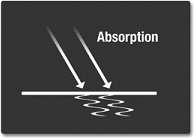
As previously described, the light may be absorbed. This happens when the light photons (or "packages") hit an object, and it's molecules are shaken up by the light. This causes the material to become hotter.
Reflection 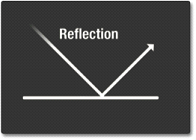
Reflection occurs when light bounces off of a material. The types (wavelengths) of light reflected depend on the composition of the material.
Diffraction 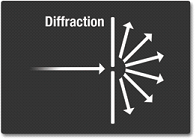
Diffraction occurs when light is bend and spread around an object or a slit.
Scatter 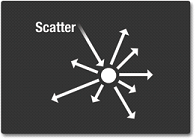
Scattering occurs when light bounces of an object in a variety of direction. The sky is blue due to light scattering. The higher energy (shorter wavelength) light (violet and blue) tends to be scattered by the atmosphere, while the longer wavelength light (yellow and red) does not. Therefore blue and violet and scattered, and the sky appears blue (more blue than violet because human's eyes are more sensitive to blue).
Refraction 
Refraction occurs when light changes direction when passing through an object. The different wavelengths are changed at different rates and allows colors to be separated The previous examples of a glass prism and rain drops are examples of refraction.
In this activity, students look at the spectrum of air, nitrogen and oxygen, and establish that these are the major constituents of air because the combined spectra of oxygen and nitrogen look like the spectrum of air. This is, of course, a very simplified "model" of the atmosphere, but enough to give students an idea of how the atmospheric constituents are established.
Before the Activity
- Complete the Using a Fancy Spectrograph activity.
- Set up light sources.
- Make copies of the Spectral Mystery Worksheet, one per person.
- Label the gas discharge tubes with their names, but label the duplicate tube "mystery."
- Turn off the classroom lights.
With the Students

- Tell students that their job as engineers today is to establish what makes up the air we breathe and, also, to figure out the composition of the "mystery" tube. They will use the spectrographs they built in the "Building a Fancy Spectrograph" activity.
- Set up the nitrogen gas discharge tube in the power supply.
- Have small groups of students look at nitrogen and draw pictures of the spectrum.
- Set up the oxygen gas discharge tube in the power supply.
- Repeat Step 3 with oxygen.
- Set up the "other" gas discharge tube.
- Repeat Step 3 with "other."
- Set up the air gas discharge tube.
- Repeat Step 3 with "air."
- Set up the mystery tube.
- Repeat Step 3 with the "mystery" tube.
- Have students figure out what air contains.
- Lastly, have the students try to figure out what is contained in the "mystery" tube.
Vocabulary/Definitions
absorption spectrum: Dark lines that appear against the continuous spectrum seen through a spectrograph.
continuous spectrum: The rainbow that white light is composed of that can be seen through a spectrograph.
diffraction: When light bends, as through a prism or diffraction grating.
diffraction grating: Usually a piece of film designed to act like a prism.
emission spectrum: Bright lines that appear through the spectrograph against a dark background.
incandescent light bulb: A standard light bulb found in most homes.
light source: Any object that produces light.
spectrograph: A tool that allows the components of light to be seen easily with the eye. (also: spectroscope)
spectrum: The pattern light produces as can be seen through a spectrograph. (plural: spectra)
Assessment
Pre-Activity Assessment
Think-Pair-Share: Ask students to share with a peer about how scientists and engineers determine what an atmosphere of a planet contains. Have a few groups share their ideas with the class.
Activity Embedded Assessment
Class Discussion: Ask students what astronomers might gain from understanding the atmospheres of other planets. Reinforce the idea that without engineering technology such as spectrographs, scientists would be unable to determine what a planetary atmosphere contains. Ask students to brainstorm about the limitations and advantages of their spectrograph now that they have used them to look at a variety of gas tubes.
Worksheet: Have students complete the activity worksheet; review their answers to gauge their mastery of the subject matter.
Post-Activity Assessment
Worksheet Discussion: Review and discuss the worksheet answers with the entire class. Use their answers to gauge students' mastery of the subject matter.
Poster Presentation: Have groups of students draw posters of the types of equipment, materials and other resources (such as people) that must be developed by engineers before sending a spectrograph into space. After 10 minutes, have groups present their posters to the class.
Safety Issues
- Gas discharge tubes get very hot. They should only be removed by a teacher.
Troubleshooting Tips
Colorblind and vision-impaired children will have difficulty with this lab. Students with corrective lenses will not have difficulty. Pair colorblind students with another student to assist with the activity and homework.
Students may need assistance adjusting the position of the grating so that a spectrum appears. The lid must be rotated if a spectrum is not visible.
Make sure all gas discharge tubes have been tested prior to the activity to confirm that they are operational.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students use the spectrograph from the "Building a Fancy Spectrograph" activity to gather data about different light sources. Using the data, they make comparisons between the light sources and make conjectures about the composition of these sources.
References
Grastang, Roy, Stern, Raul, Robertson, Scott, et.al. ASTR 1010 Laboratory: Introduction to Astronomy. 1987/2005. Keith Gleason/Sommers-Bausch Observatory, University of Colorado. 10/2006 http://carbon.ucdenver.edu/cmesa/wired/documents/curriculum/h%20-%20Project%20SPECTRA/Teacher%20Guide.pdf
Copyright
© 2007 by Regents of the University of Colorado.Contributors
Laboratory for Atmospheric and Space Physics, University of Colorado at BoulderSupporting Program
Laboratory for Atmospheric and Space Physics (LASP), University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education, and National Science Foundation GK-12 grant no 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: May 14, 2019



User Comments & Tips