Summary
Students use a recipe to prepare a hydrogel gummy snack, which has a similar consistency to that found in a Haribo® gummy product. They must convert the juice and gelatin-based recipe from US customary units to metric units with dimensional analysis conversion. After unit conversion, teams are given different gelatin quantities and design their gummy snacks. Once the candies have solidified, student groups compare the gummy snacks are for viscosity and taste. After a taste test, teams reflect on their experiment and brainstorm ways to iterate a better gummy recipe.
Engineering Connection
Food, industrial, and chemical engineers may work with food products to help enhance production techniques. Food engineers may help design new types of foods based on existing technologies to make the products we consume last longer. Industrial engineers may identify ways to preserve the shelf-life of packaged foods. Chemical engineers may work on ways to enhance the taste of certain types of foods, so a product has a uniform taste off the production line. Food manufacturing companies employ engineers for a variety of reasons. In this activity, students become food engineers to engineer the perfect gummy snack!
Learning Objectives
After this activity, students should be able to:
- Observe how chemical properties apply to changes in matter, particularly in food production.
- Apply math to unit conversions to build hydrogel structures.
- Understand what kind of parameters are important in preparing chemical solutions.
- Prepare a chemical solution.
- Communicate and present solutions based on evidence.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS1-6. Refine the design of a chemical system by specifying a change in conditions that would produce increased amounts of products at equilibrium. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Refine a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms. Alignment agreement: In many situations, a dynamic and condition-dependent balance between a reaction and the reverse reaction determines the numbers of all types of molecules present.Alignment agreement: Criteria may need to be broken down into simpler ones that can be approached systematically, and decisions about the priority of certain criteria over others (trade-offs) may be needed.Alignment agreement: | Much of science deals with constructing explanations of how things change and how they remain stable. Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-ETS1-4. Develop a model to generate data for iterative testing and modification of a proposed object, tool, or process such that an optimal design can be achieved. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to generate data to test ideas about designed systems, including those representing inputs and outputs. Alignment agreement: | Models of all kinds are important for testing solutions. Alignment agreement: The iterative process of testing the most promising solutions and modifying what is proposed on the basis of the test results leads to greater refinement and ultimately to an optimal solution.Alignment agreement: | |
Common Core State Standards - Math
-
Compute unit rates associated with ratios of fractions, including ratios of lengths, areas and other quantities measured in like or different units.
(Grade
7)
More Details
Do you agree with this alignment?
-
Recognize and represent proportional relationships between quantities.
(Grade
7)
More Details
Do you agree with this alignment?
-
Reason quantitatively and use units to solve problems.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the role of troubleshooting, research and development, invention and innovation, and experimentation in problem solving.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Explain how technology and engineering are closely linked to creativity, which can result in both intended and unintended innovations.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Analyze examples of technologies that have changed the way people think, interact, and communicate.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Texas - Math
-
convert between measurement systems, including the use of proportions and the use of unit rates.
(Grade
7)
More Details
Do you agree with this alignment?
Texas - Science
-
in all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical reasoning, and experimental and observational testing, including examining all sides of scientific evidence of those scientific explanations, so as to encourage critical thinking by the student;
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
use appropriate tools to collect, record, and analyze information, including life science models, hand lens, stereoscopes, microscopes, beakers, Petri dishes, microscope slides, graduated cylinders, test tubes, meter sticks, metric rulers, metric tape measures, timing devices, hot plates, balances, thermometers, calculators, water test kits, computers, temperature and pH probes, collecting nets, insect traps, globes, digital cameras, journals/notebooks, and other equipment as needed to teach the curriculum; and
(Grade
7)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 75 ml potable water
- 50 ml juice, such as sugar-free beet, orange, and apple
- 6 g unflavored Knox gelatin; 473 ml (16 oz.) containers are available online
- 75 ml graduated cylinder
- 50 ml beaker
- 0.5-1 cm magnetic stirring bar (coffee straws can work in a pinch)
- magnetic stirrer with hot plate attached to a temperature probe, or hot plate with clean thermometer or temperature gauge; examples available online
- flat Styrofoam plate
- small coffee filter
- plastic teaspoon
- silicone candy molds, available online
- plastic candy dropper (to transfer solution from beaker to mold)
- paper towels for clean-up
- digital scale (that can measure in both grams and ounces)
To share with the entire class:
- ice in cooler or a refrigerator
- gloves
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/rice-2371-engineering-gummy-candy-hydrogels] to print or download.Pre-Req Knowledge
For math, students should have skill and understanding of:
- multiplying and dividing
- rounding numbers
- quantities
- unit analysis
- calculator use
For chemistry, students should understand states of matter and solutions including knowledge of solvents, solutes, and solutions. (See the Engineering the Perfect Gummy Candy Presentation for more on states of matter.)
Introduction/Motivation
(Incorporate the Engineering the Perfect Gummy Candy Presentation into your introduction.)
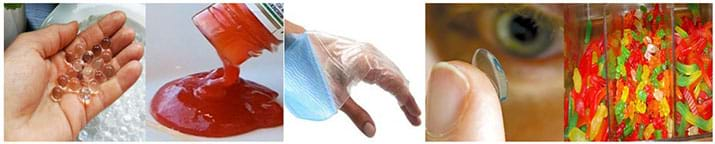
Today you will become food engineers and create your own hydrogel! So what, exactly, is a hydrogel? Hydrogels are prepared with suspended particles, or solutes, that maintain their solid characteristics while in a solution made primarily of water as its primary component. All hydrogels require the following: a proper measurement of ingredients, proper ratio of ingredients, and a specified mixture sequence to ensure the hydrogel has the proper consistency and viscosity.
Although the term hydrogel sounds very scientific and new to most people, they are routinely used in food. Can you guess what kind of foods that might actually be hydrogels? (Potential answers: Jell-O, gummy worms or bears.)
Jell-O and gummy candies are two popular, edible hydrogels. The solutions of Jell-O and gummy candies are prepared in a similar way to hydrogels that are produced for medical purposes, such as lotions, gel pads, or contact lenses. Whether it is a science experiment or for cooking, hydrogels must be prepared using exact ratios of solvents and solutes.
Today you will aim to make the perfect hydrogel in the form of a gummy candy! You have all the ingredients, but you need but you need to determine how much solution you need to prepare to fill your silicone mold. In a similar manner, engineers routinely use conversion factors to adjust the yield and portions of a particular product.
Why do we start with determining the weight and volumes of the ingredients to be used per mold? (Answer: We want to make sure that we have enough ingredients before beginning the activity.)
Procedure
Background
Although the term hydrogel sounds scientific, they are routinely prepared using gelatin (a type of collagen protein) and various liquids. Hydrogels are used in popular food products such as ketchup, Jell-O and gummy candies. Solutions used to manufacture edible hydrogels are applied in a similar fashion to hydrogel solutions used in medical applications, such as contact lens or lotions. Hydrogels contain suspended particles (solute) that maintain their solid characteristics while in a solution using a liquid made up of primarily water as its primary solvent component.
How do we define a hydrogel? A hydrogel is a viscous substance in which the liquid component is water and the solid component is a protein. It holds large quantities of water in a three-dimensional lattice by encasing solids surrounded by cross-linked junctions. Hydrogels can occur both naturally, such as in animal collagen found in connective tissue, and synthetically such as gelatin, which is derived from connective tissue or bones, but often exists in a powdered state.
In this activity, student teams are challenged to scale a quantity of gelatin that will allow them to make a gummy candy. The original recipe uses US customary units. Students should convert the customary units of cups and ounces to metric units of milliliters and grams. Students learn how to change the ratio of components to scale the final solution volume up or down in order to prepare the hydrogels. The engineering design process can be incorporated by allowing the students to decide on what quantity of gelatin will change the viscosity, and ultimately the taste, of their edible hydrogel. Is more gelatin better? Or, are their higher or lower limits to the known recipe’s quantity of gelatin?
Known Proper Ratio:
- 236.9 ml (1 cup) pure 100% juice (with no sugar added; see Troubleshooting Tips)
- 28.3 g (2 tbsp) unflavored gelatin
Before the Activity
- Copy and distribute the pre-activity (or provide as homework): Hydrogel Wordfind
- Explain the activity using the 5E Approach
- Engage: Introduce concepts of hydrogels and show a portion of a YouTube video (see Additional Multimedia Support)
- Elaborate: Conversion Between US Customary Units & Metric Systems Sheet
- Explain: Engineering the Perfect Gummy Candy Presentation
- Explore: Activity – Preparing Gummy Candy
- Evaluate: Conversion Factor to Adjust Final Yield Sheet
- Determine the approximate range in time to warm the juice to 30°C and then 45°C on the heating plate.
- Decide the number of molds per teams. Groups can share a mold but they would need to prepare solutions at the same time.
With the Students
- Determine volume for mold. Pour 75 ml of potable water into the graduated cylinder. Use the candy eye dropper to transfer the water into each section of the mold. Calculate the volume needed to fill the mold by subtracting the remaining volume in the cylinder from 75 ml.
- Calculate conversion factor. Calculate the conversion factor to determine the volume needed to fill the silicone mold for the hydrogel. Use the worksheets, Conversion Between US Customary Units & Metric Systems and Conversion Factor to Adjust Final Yield.
- Weigh ingredients. Weigh the calculated quantity of gelatin and pour the volume of juice to use.
- Prepare a solution of the gummy candy. Pour the juice into a 50 ml beaker. Add a magnetic stirring bar to the beaker. Begin rotating the bar and heating (see Figure 1). When temperature reaches ~30°C, slowly add a quarter to no more than half of plastic teaspoon of gelatin into the juice while gently stirring until the gelatin is fully dissolved. Repeat until all gelatin is added and dissolved. Heat the juice to final temperature of ~37°C - ~48°C. Note: Heat the juice until it’s very warm (~37°C - ~48°C) but not boiling. If the liquid is too hot, it could break down the gelatin protein and your gummies may not set. Add the gelatin powder into the warm juice.

- Prepare the gummy mold. Place the silicone mold on top of a flat paper plate. Once in the gummy solution is mixed thoroughly, use the plastic dropper to add the solution into the silicone candy molds.
- Quick chill. Carefully place the molds flat on a top of ice in a cooler to chill for about 20 minutes. Or, position the molds on a paper plate and place in a refrigerator at 0-4°C for 20-40 minutes. Gels can remain overnight to several days in a refrigerator.
- Test viscosity. Place mold over Styrofoam plate and turn mold upside down until Gummy snack slips out. The proper ratio of gelatin to juice will result in a suspension of hydrogel that does not “drip” or stay stuck to the mold.
- Taste test. Take turns trying the gummy candy. Is it too dilute (weak) or too concentrated (strong)?
- Iterate. Brainstorms for ideas on how to make a better hydrogel based on the tests above. What happens if students add more gelatin or use less juice?
- Repeat steps 1 through 6. Iterate if time permits to emphasize the importance of improving upon the product. Have students try measuring adding more gelatin, or less, and compare results.
- Compare and taste. Teams can compare viscosity and taste resulting from changes in gelatin concentration with the original gummy recipe. Viscosity can be tested by removing a gummy product from the mold. If it is too fluid, the product will not have solidified due to low gelatin (solute) not forming a scaffold to hold the juice (solvent). Compare the original recipe to the iterative recipe with higher gelatin concentration with a touch test to feel and see the differences in viscosity.
- Present. Have students complete the Student Team Response Sheet and present their findings in groups.
Vocabulary/Definitions
conversion factor: A numerical quantity to multiply or divide, or a number to convert to an equivalent unit.
dimensional analysis conversion: A method for converting one unit to a different unit; also known as factor-label method or unit-factor method.
gelatin: A protein derived from collagen, usually collected from cow or pig bones, skin, or connective tissue.
hydrogel : A solidified gel in which the primary liquid component is water.
parameter: Similar to a variable in that the value also varies (but is normally defined as being within a certain area); a 'link' between two other variables.
solute: A substance that is dissolved within a solvent, forming a solution.
solution : A liquid mixture in which the minor component (the solute) is uniformly distributed within the major component (the solvent).
solvent: Able to dissolve other substances.
viscosity: Thick, sticky, and semi-fluid in consistency; fluid (liquid or gas) that opposes flow or resists change in shape.
Assessment
Pre-Activity Assessment
Hydrogel Wordfind: Have students work through the Hydrogel Wordfind.
Activity Embedded Assessment
Conversion Sheet: Have students work on the Conversion between US Customary Units & Metric Systems Sheet.
Conversion Factor: Have students review and complete Conversion Factor to Adjust Final Yield Sheet.
Post-Activity Assessment
Student Team Response Sheets: Have students complete the Student Team Response Sheet.
Investigating Questions
- At the end of the class, have students share, compare, and reflect on what worked and what was challenging about the activity.
- Was making the conversion factor challenging?
- What were the challenges in deciding whether to change the volume of juice or the gelatin quantity?
- Which ratio of juice to gelatin produced a firm hydrogel?
- Which hydrogel tasted the best?
- If you prepared these hydrogels again, what would you change?
- Why does the volume (size) of the mold alter solidification time?
Safety Issues
- If students are to eat the final product, all ingredients must be new and glassware items should be cleaned with commercial dish soap. Assuming students will eat the final products, ask students to clarify if they have any food allergies. Students should use gloves while preparing their hydrogels. Other lab safety measures, (aprons, goggles, closed-toed shoes, etc.) are not necessary, but may help introduce students to the lab environment.
Troubleshooting Tips
- Silicone molds: Determine how many individual portions per mold will be made from the original recipe. For example, a volume of 50 ml filled the small gummy bear shapes, while volumes of 75-95 ml were needed to fill the larger star and shell shapes.
- Juice: Use sugar-free beet, apple, pomegranate, or orange juice. Do not use pineapple, mango or papaya juice as they contain an enzyme called bromelain that breaks down gelatin.
- Gelatin concentration: 4-6 g gelatin per 50 ml juice solidifies within 20-40 minutes on ice (not in ice) or in the refrigerator. <3 g gelatin per 50 ml does not solidify.
- Bubbles: Avoid air bubbles during the transfer of solution to mold. To reduce air bubbles, squeeze the candy dropper’s bulb prior to and while placing into beaker. Slowly place dropper tip towards middle of fluid in the beaker (not at top of fluid). Release pressure slowly and allow fluid to move up candy dropper. Do not allow fluid to rise into bulb.
- Solidifying time: Solidification can occurs within 40 minutes; however > 2 hours is recommended. Gummy candy refrigerates for up to two weeks without an issue. The time needed for solidification increases with the increasing size of silicone mold’s shapes as well as the temperature of the solution.
Activity Scaling
- For middle school, demonstrate all steps of the unit conversion as a guided activity followed by students copying the calculations. Students can check their answers using an online conversion calculators (see Conversion between US Customary Units & Metric Systems Sheet Answer Key).
- For high school, students can complete the unit conversion worksheet. Students can calculate by hand or by using Microsoft Excel. Students can check their answers using online conversion calculators. (See Conversion between US Customary Units & Metric Systems Sheet Answer Key.)
- Assign students to write a paper about engineering hydrogels in the food industry or for biomedical applications.
Additional Multimedia Support
There are numerous videos about hydrogels and their applications. YouTube videos are included in the last slide of the PowerPoint. Teachers can use a video as the beginning (the Engage of a 5E Lesson Plan) and then use one or two videos after the activity and the student discussion (the Elaborate of a 5E Lesson Plan). The Biomaterials Lecture (below) is most likely too long and advanced to show in class, however some of the slides offer insight into the science of hydrogel material.
Kara Spiller. Biomaterials Lecture - Natural polymers and hydrogels (2016)
https://www.youtube.com/watch?v=gxGeK4rzEr0
Mitch Plumley. Hydrogel Polymers (2018)
https://www.youtube.com/watch?v=BE1xk1rlrGg
ALIwebsite. Occupational Video - Food Scientist
https://www.youtube.com/watch?v=tweElJtj3o8
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students use sodium alginate to create hydrogels that have cross-linking ability, biocompatibility, chelating ability, water solubility, and low cost. These alginate-based hydrogels are also nontoxic and non-inflammatory. All these properties make the alginates ideal for applications in pharmaceutic...
Copyright
© 2019 by Regents of the University of Colorado; original © 2018 Rice UniversityContributors
Jodie PolanSupporting Program
Engineering Research Center for Nanotechnology Enabled Water Treatment Systems (NEWT) RET, Rice UniversityAcknowledgements
This material was developed based upon work supported by the National Science Foundation under grant no. EEC 1406885—the Nanotechnology Research Experience for Teachers at the Rice University School Science and Technology in Houston, TX. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
This activity was inspired by a 2018 NSF summer research program in the laboratory of Jeffrey Hartgerink, PhD (Depts Biomedical Engineering and Chemistry, Rice University). I appreciate the research training from his graduate students Cheuk Sun Edwin Lai, David Leach and Nicole Carrejo. His entire laboratory staff made learning to prepare protein-based hydrogels for biomedical applications informative and fun. Special thanks to Isaias Cerdas, Christina Crawford & Carolyn Nichol, PhD in the Rice RET-STEM office for administrating the program and arranging STEM educational field trips.
Last modified: May 20, 2025




User Comments & Tips