Quick Look
Grade Level: 11 (10-12)
Time Required: 5 hours
(six 50-minute class periods + additional time outside of class for students to work independently)
Expendable Cost/Group: US $5.00 This activity also uses many non-expendable (reusable) items see the Materials List.
Group Size: 3
Activity Dependency: None
Subject Areas: Chemistry, Science and Technology
NGSS Performance Expectations:

| HS-ETS1-2 |

Summary
Students are introduced to polymer science and take on the role of chemical engineers to create and test a plastic made from starch. After testing their potato-based plastic, students design a product that takes advantage of the polymer’s unique properties. At the end of the engineering design process, students present their product in a development “pitch” that communicates their idea to potential investors.Engineering Connection
A fundamental concept materials and chemical engineers must understand is why a change in the chemical structure of polymer molecules affects the properties a plastic. Engineers use concepts based in the scientific understanding of polymers to design a variety of products made with plastics. Along with testing and designing new plastics, engineers are often tasked with communicating their results to potential stakeholders. Engineers must take known methods of a design and reconstruct or redesign them in order to meet the needs of a particular company or investor.
Learning Objectives
After this activity, students should be able to:
- Explain the basics polymer formation and their characteristics.
- Apply the engineering design process in order to address a real-world challenge.
- Conduct research to support and improve upon a design.
- Communicate results and the importance of a particular design.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
Communicate scientific and technical information (e.g. about the process of development and the design and performance of a proposed process or system) in multiple formats (including orally, graphically, textually, and mathematically).
(Grades 9 - 12)
More Details
Do you agree with this alignment?
-
Investigating or designing new systems or structures requires a detailed examination of the properties of different materials, the structures of different components, and connections of components to reveal its function and/or solve a problem.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-2. Design a solution to a complex real-world problem by breaking it down into smaller, more manageable problems that can be solved through engineering. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Design a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | Criteria may need to be broken down into simpler ones that can be approached systematically, and decisions about the priority of certain criteria over others (trade-offs) may be needed. Alignment agreement: | |
Common Core State Standards - English
-
Integrate and evaluate multiple sources of information presented in diverse formats and media (e.g., quantitative data, video, multimedia) in order to address a question or solve a problem.
(Grades
11 -
12)
More Details
Do you agree with this alignment?
-
Evaluate the hypotheses, data, analysis, and conclusions in a science or technical text, verifying the data when possible and corroborating or challenging conclusions with other sources of information.
(Grades
11 -
12)
More Details
Do you agree with this alignment?
-
Synthesize information from a range of sources (e.g., texts, experiments, simulations) into a coherent understanding of a process, phenomenon, or concept, resolving conflicting information when possible.
(Grades
11 -
12)
More Details
Do you agree with this alignment?
Common Core State Standards - Math
-
Summarize, represent, and interpret data on a single count or measurement variable
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of engineering design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of the role of troubleshooting, research and development, invention and innovation, and experimentation in problem solving.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop abilities to apply the design process.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Mississippi - Science
-
Apply inquiry-based and problem-solving processes and skills to scientific investigations.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 100 g potatoes (the variety doesn’t matter)
- 250 mL beaker
- large watch glass, available online
- hot plate
- petri dish
- pH indicator strips (approx. 5-10 with testing)
- disposable pipettes (approx. 4-5)
- stirring rod
- 25 mL graduated cylinder
- 10 mL graduated cylinder
- goggles
To share with the entire class:
- vegetable grater
- food processor (at least one per class, but one for each group is preferred)
- tea strainer or mesh strainer or cheese cloth (at least one per class, but one for each group is preferred)
- 100-150 mL bottle of glycerol
- 100-150 mL bottle of 0.1 M hydrochloric acid
- 100-150 mL bottle of sodium hydroxide
- distilled water
- an assortment of plastics items such as water bottles, plastics bags, containers, and PVC
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/usm-2287-engineering-polymers-potatoes] to print or download.Pre-Req Knowledge
Students should have a basic knowledge of:
- Covalent and ionic bonding
- Acids and bases
- Monomers
- How to calculate density
- Boiling and melting points
- Basic lab equipment and safety knowledge
Introduction/Motivation
[Be ready to show “From DNA to Silly Putty, the Diverse World of Polymers” video (4:59 long)]
What happens when a researcher or engineer develops a product for a specific purpose, only to see it founder or, even worse fail? As the saying goes, “if at first you don’t succeed, try, try again.”
Or, in the language of engineers, you iterate! If you’ve ever molded something with Play-Doh or used Super Glue as an adhesive, you are using a product that was designed for an initial purpose, only to be reinvented for a completely different use. Play-Doh was originally invented as a putty that could clean coal residue from wallpapers, but was repurposed as a modeling clay. Super Glue (known by its scientific name as cyanoacrylate) was initially designed as a plastic for use in gun sights during World War II, but was redesigned and repurposed as the fast-acting adhesive that we use today.
In this activity, you will also play the role of an engineer by using existing knowledge in new and unique ways to make a desired product. You will research and choose the product in which you want to improve. The only parameter you have is that you have to develop your own polymer – a type of plastic.
After developing your product, your task will be to communicate why you designed and iterated upon your material in a presentation similar to “Shark Tank!”
[You may go into the basics of polymer science with this introductory video focusing on discovering what a polymer is made of and the unique/useful properties they can have: “From DNA to Silly Putty, the Diverse World of Polymers”]
What from the video have you learned about polymers? [Answers will vary.] The main idea is that polymers are long chains of repeating molecules. Do you have any examples of other polymers you have encountered?
[Answers will vary. Suggested answers include: plastic bottles, nylon, Silly Putty, DNA, carbohydrates such as starch and cellulose, Styrofoam, finger nails, exoskeletons of animals, rubbers, plastics, proteins, PVC pipe; elaborate on their examples and as more as needed, emphasize that the main thing you want them to understand is how common, beneficial, and diverse polymers can be.]
If polymers are so useful does that mean they are the perfect resources?
[Answers will vary. Suggested answers include: Not necessarily, because most synthetic polymers are manufactured with petroleum which is a non-renewable resource. Also, some polymers such as Styrofoam, plastic bags, and plastic bottles are not as recyclable or are not recycled so they fill up landfills or the ocean, which hurt our environment.]
As you can see polymers are certainly compounds with many uses. As we move into the activity, let’s also consider that while they have many benefits to our society, we should also consider how polymers are produced and their impact on our environment.
Procedure
Before the Background and Introductory Lab
- Make a copy of the following handout for each student:
- Have supplies ready for this lab (see the Materials List).
- Have all materials and equipment ready per group.
Before the Post-Lab
- Make a copy of the following handout for each student:
- Print and cut out (and laminate, if possible) the Polymer Structure Cards. These should be placed in stations around the room where the students can access them.
- Have various plastics items like water bottles, plastics bags, containers, PVC, etc. collected in one area.
- Prepare to show the 10:14 long video, “Polymers – Crash Course Chemistry #45” [https://www.youtube.com/watch?v=rHxxLYzJ8Sw].
Before the Project Directions/Elaborate
- Make a copy of the following handouts for each student:
- Students will need the completed form in Engineering Polymers from Potatoes Lab Packet.
With the Students
Part 1: Background and Introductory Lab
- Give each student an Engineering Polymers from Potatoes Lab Packet and have them complete the pre-lab/discussion questions with their lab groups. Students should read through the procedure and use the structural guides included in the packet to answer the questions. Review answers to questions as a class and clarify any terms they listed that they do not know from the procedure.
- Pass out all supplies to students and instruct them on how to handle items (such as the food processor, grater or tea strainer) that are to be shared with classmates throughout the lab. Also, review relevant lab safety precautions.
- Instruct students to begin following the procedure for Part 1: Extracting the Starch. To extract the starch, have students clean a potato, place a plate on a balance and zero (tare weight) it out, then grate the potato onto the plate until they have about 100 g. Have students place their potato pieces into a food processor (blenders also work well), add about 100 mL of distilled water and process the potato for a few seconds. Navigate groups taking turns if you are limited in your number of food processors; to speed up the process groups can pour their contents into a separate container as soon as they are done with the processor.
- Next, students will pour the liquid portion of their mixture through cheesecloth into a separate beaker, leaving the potato behind.
- With the leftover potato they will repeat the process of adding water, processing it, and pouring the liquid through the cheesecloth adding it to the liquid they collected previously. Have the students allow the mixture to settle for five minutes, then they will decant (many may not know what decant means, so it may have to be explained or modeled to them) the water from the beaker leaving behind the starch (this will show as a layer of white at the bottom of the beaker).
- Students will add another 100 mL of distilled water and stir gently, letting it settle again and decanting a final time. The final white substance will be the starch they will use in Part 2. This is a good place to break if you do not have time to complete Part 2 in the same class period.
Part 2: Making the Plastic
- Have students measure 25 mL of distilled water in a graduated cylinder and pour into a 50 mL beaker; then add 2.5 g of their potato starch from Part 1, 3 mL of 0.1 M hydrochloric acid, and 2 mL of glycerol.
- Next, have the students place their mixture on a hotplate with a watch glass on top. They will carefully bring the mixture to a boil and let it boil gently for 15 minutes. Instruct students to make sure not to let the mixture boil until the solution dries out and to lower the heat if it looks like that is a possibility.
- After the 15 minutes have the students use a glass rod to test the pH of the solution by dipping it into the mixture and onto a piece of indicator paper. The paper should indicate an acid solution. Students should then add 0.1 M sodium hydroxide slowly (few drops at a time), testing with the stirring rod and pH paper after each addition until the mixture indicates a neutral solution. This should take about 3 mL of sodium hydroxide.
- Finally, have the students pour the mixture into a labeled petri dish and rotate the dish to get an even covering, letting it sit uncovered to dry out. The drying process usually works overnight, as another option the samples can be placed in an oven to dry out quicker.
- Students will repeat steps 7-12, but without using the glycerol to compare. If time is an issue you can assign some group to carry out the procedure with glycerol and some without rather than having each group do both.
Part 3: Testing the plastics
- Once their plastic has dried, instruct students on how to run testing and have them fill in the data table on page 3 in their Engineering Polymers from Potatoes Lab Packet.
- To determine the density, have the students cut a piece of their plastic (2 cm x 2 cm) and find the mass of the piece. Instruct students to use a graduated cylinder determine the volume of that piece via displacement using the equation d = m/v. (This may not be applicable if the plastic is too gel-like.)
- To determine the melting properties students can place a 2 g sample of their plastic on a watch glass and heat it on a hotplate. This can be done qualitative where they describe if/how the plastic burns or quantitative if they can measure the point at which the plastic begins to melt.
- Additional qualitative testing can be done following the questions and chart in their lab packet.
Post-Lab/Explain
- Once all groups have had a chance to collect the data discuss with the students their results including comparing the glycerol product vs. the product without glycerol, the purpose of the acid, heating, and adding the base, etc. Ask the students, “What products the glycerol product could be used for [answers will vary] and what the non-glycerol product could be used for [answers will vary]?”
- Give each student a Crash Course Video Guide and instruct them to fill in the chart as they watch the video with key concepts and pictures related to each term. Play the video “Polymers – Crash Course Chemistry #45” [https://www.youtube.com/watch?v=rHxxLYzJ8Sw]. After the video, have students share what they wrote or included for each term.
- Direct students attention to your examples of various plastic products. Place the Polymer Structure Cards around the room and instruct the students to work together to the plastics next to the structure they think best fits their function based on what they have learned so far about polymers. When they are done discuss each group and the similar properties of their plastics and their functions. Relate each one to what they observed in the plastics they made with the potato with and without glycerol.
Project Directions/Elaborate
- Explain to students in the next phase of this activity they will be working as chemical engineers to create, and test a plastic made from starch a starchy food of their choice.
- Instruct the students that their first steps are to research different starchy products to help direct them towards a particular food to start with, rather than just randomly picking something up at the store. Here you can give them the How to do Research Handout and if possible spend a class period helping them conduct research. Also distribute the Shark Tank Project Overview sheet.
- Instruct the students that they will use the original procedure from the Engineering Polymers from Potatoes Lab as a guide to making their own starch. However, they will choose how much glycerol, acid, or base to be added/removed from the original procedure. Additionally, they will determine the proper heating time, etc.
- Once they make their own plastic, students will determine a use of that plastic that they can then present to a panel of “sharks” like an episode of Shark Tank. [A homework option is to have them watch an episode of Shark Tank and give a summary/report of what was included in the products that were successful and how they compared to ones that weren’t, etc.]
- Clearly explain to students that prior to going into the lab for revisions, they must have:
- a thorough procedure written about the how they chose to make and test their plastic,
- evaluations/reviews from two classroom peers [Procedure Peer Evaluation handout] Note: This can be done in class if time permits or can be assigned as homework to be completed by a particular date
- and lastly approval by you
- Be sure to pass out the Procedure Peer Evaluation.
- Explain that once they have created their plastic, they then can do additional research and come up with ideas about how their plastic can be used based on its properties. Once the product has been determined, they can begin to put together their proposal and presentation by researching additional factors such cost, environmental concerns, etc. Note: Please make it clear to students that just because a polymer does not turn out as they expected does not mean it is wrong or bad, but as part of the engineering process they must determine what it could be used for based on its unique properties.
- The last aspect of their project will be a typed scientific article following the criteria in the Shark Tank Project Overview sheet where they elaborate on their findings and report on their product.
- Determine the due dates of each part of the assignment; the order of the presentation and the article can be flipped. For your “sharks” you may consider recruiting instructors from local universities, community members, staff, administration, etc. Motivate the students to get creative by coming up with jingles, samples and logos to impress the sharks!
- Present their research and pitch to the “sharks”. The sharks will judge the projects via the criteria listed on the Polymer Shark Tank Rubric. Note: For those students who need more guidance, have students add an additional step of “meeting with a shark” in their Procedure Peer Evaluation and use this step to give students more direction to help with their revisions.
Vocabulary/Definitions
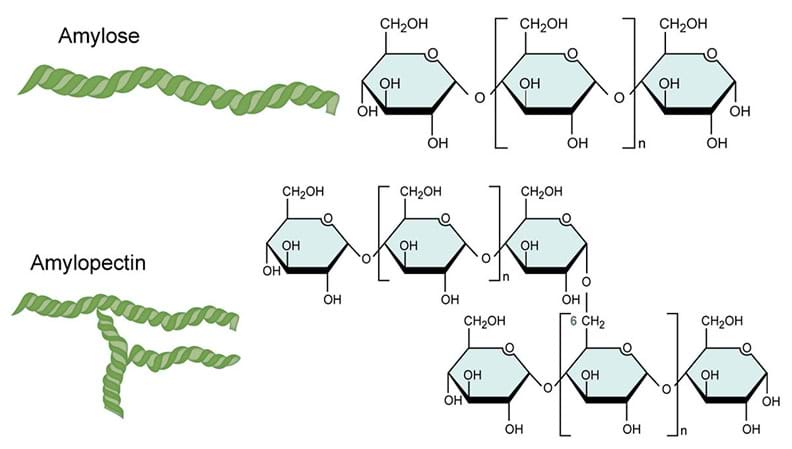
amylopectin: Form of starch consisting of branched polysaccharide chains.
amylose: Form of starch consisting of long unbranched polysaccharide chains.
glycerol: A colorless, sweet, viscous liquid used widely in the food and pharmaceutical industries. It may be used as a sweetener, lubricator, or a moisturizer.
hydrochloric acid: A colorless inorganic chemical system with the formula H2O:HCl. It has a distinctive pungent smell and is classified as strongly acidic. Hydrochloric acid is the simplest chlorine-based acid system containing water.
monomer: A molecule that can be bonded to other identical molecules to form a polymer.
polymer: Compounds formed by repeating structural units called monomers.
polysaccharide: A carbohydrate whose molecules consist of a number of sugar molecules bonded together.
sodium hydroxide : Also known as lye and caustic soda, it is base and alkaline, inorganic compound with the formula NaOH.
Assessment
Pre-Activity Assessment
Discussion questions: Gather an understanding of students’ prior understanding of polymers, plastics, and engineering by asking the following questions:
- What is a polymer, can anyone give some examples? Something made from putting many small molecules together; nylon, molecules of DNA, cellulose, PVC
- What are plastics and what are some examples of their uses? Malleable material made of polymers; Examples include PVC pipes, 2-liter/water/soda bottles, hair product bottles, cell phone cases, plastic wrap, etc.
- What role do engineers play in developing products? What is the engineering design process? Engineers analyze and evaluate real world problems to design, test, or improve upon a solution. The engineering design process are the steps that engineers take to construct a solution to address a problem. During this process, engineers get to design, build, and test their product, as well as make modifications when necessary to meet a goal.
Engineering Polymers from Potatoes Pre-Lab: Students use information from structural image guide and procedure to answer questions about the lab. Review questions as a class prior to starting the lab. (Suggested answers are in italics)
- Explain/describe what a polymer is. A polymer is a substance that is made of many repeating subunits called monomers.
- What characteristics make starch a polymer? How is it held together? Starch is a polymer, because it is made of many subunits of glucose bonded together. Starch is held together by hydrogen bonds between the glucose monomers.
- Identify the amylopectin and amylose sections of the starch molecule. How are they different? Amylose is a linear strand, and amylopectin is a branched strand. Amylopectin is more complex.
- Describe how the propan-1,2,3-triol connects to the starch molecule? Propan-1,2,3-triol connects between the linear chains of the starch molecule.
- Read through the procedure and list all materials and safety precautions you will need:
- List any words you do not know/recognize in the procedure:
Activity Embedded Assessment
Crash Course Video Guide: After showing the Crash Course Polymer video have students share what terms, words, descriptors, etc. they associate with each of the key terms provided on the Crash Course Video Guide.
Peer Evaluate Procedure: Lead students in peer evaluating the procedure they came up with to make their polymer, making sure to focus on having enough detail and reflection of the purpose of steps. Lead discussion if common errors or issues emerge. See Procedure Peer Evaluation sheet.
Post-Activity Assessment
Scientific Article: Students write a scientific article based on their research into polymers, plastics, their starch, their product, and its properties. The article will be assessed based on the following criteria*:
- Basic info/formatting
- Research
- Introduction
- Background
- Procedure
- Discussion/results
- Identify possible applications and future work ideas
- Bibliography
Pitch/Presentation: Students prepare a presentation where they pitch their product to panel of judges. They will be evaluated on the following criteria*:
- Presentation quality
- Additional visual or artistic aid
- Delivery
- Quality of product
- Validity
- Creativity
- Cost analysis & environmental impact
*Use the Polymer Shark Tank Rubric to grade student work.
Safety Issues
- Hydrochloric acid has minimal hazard at the concentration used in this activity
- Sodium hydroxide can be a mild irritant at the concentration used in this activity
- Glycerol has no hazard classification; however, it may be harmful if ingested in quantity
- Always be careful when heating substances with the hotplate
Troubleshooting Tips
Samples should dry enough overnight, but under some conditions may take longer.
Activity Extensions
Students can further develop and revise their products based on feedback from the judges.
Activity Scaling
- For lower grades, following a discussion about polymers, students may be able to assist the teacher in making the plastic as a demo and then make observations of the product. They may also provide input on the type of starch and different amounts of glycerol to create different samples for the teacher to make.
- Higher grades may be allowed greater independence in choosing their starch, researching, and experimenting with their products.
- An alternative to this activity includes giving students a real-world problem, and then having them manipulate types of starch to solve that problem. This is a more teacher-guided approach since it gives students more direction beforehand. Note you may have to change some parts of the original lesson to accommodate this approach.
Additional Multimedia Support
Polymers - Crash Course Chemistry #45 https://www.youtube.com/watch?v=rHxxLYzJ8Sw
From DNA to Silly Putty, the diverse world of polymers – Jan Mattingly https://www.youtube.com/watch?v=UwRVj9rz2QQ
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students explore the chemical identities of polymeric materials frequently used in their everyday lives. They learn how chemical composition affects the physical properties of the materials that they encounter and use frequently, as well as how cross-linking affects the properties of polymeric mater...

Students explore the basic characteristics of polymers through the introduction of two polymer categories: thermoplastics and thermosets. During teacher demos, students observe the unique behaviors of thermoplastics.

Students are introduced to the concept of viscoelasticity and some of the material behaviors of viscoelastic materials, including strain rate dependence, stress relaxation, creep, hysteresis and preconditioning. Viscoelastic material behavior is compared to elastic solids and viscous fluids.

Students learn how plastics in the human trash stream end up as microplastic particles entering the food chains via polluted water, harming animals and people. They think of ways to reuse or replace the common plastic items they discard daily. They learn how microplastics persist in the environment ...
References
Making a Plastic From Potato Starch. The Royal Society of Chemistry: Advancing the Chemical Sciences. Practical Chemistry. Accessed June 2017. http://rsc.org/learn-chemistry/resource/res00001741/making-plastic-from-potato-starch
Copyright
© 2019 by Regents of the University of Colorado; original © 2017 University of Southern MississippiContributors
Rebecca Hooper; Robin LewisSupporting Program
Research Experience for Teachers Program, School of Polymers and High Performance Materials, University of Southern MississippiAcknowledgements
This activity was developed under the Research Experiences for Teachers (RET) in Engineering and Computer Science Site for Sustainable Polymer Engineering Research program in the University of Southern Mississippi’s School of Polymers and High Performance Materials, funded by National Science Foundation RET grant no. EEC 1406753. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: March 26, 2019






User Comments & Tips