Quick Look
Grade Level: 6 (6-7)
Time Required: 1 hour
Lesson Dependency:
Subject Areas: Physical Science, Physics, Science and Technology
NGSS Performance Expectations:

| MS-PS3-5 |

Summary
Students makes sense of kinetic and potential energy, including various types of potential energy: chemical, gravitational, elastic and thermal energy. They identify everyday examples of these energy types, as well as the mechanism of corresponding energy transfers. They learn that energy can be neither created nor destroyed and that relationships exist between a moving object's mass and velocity. Further, the concept that energy can be neither created nor destroyed is reinforced, as students see the pervasiveness of energy transfer among its many different forms. A PowerPoint® presentation and post-quiz are provided.Engineering Connection
A firm understanding of energy types and energy conversion is essential to understanding the different forms of energy (and energy transfers) so common in our everyday lives, as well as a basis for comprehending more advanced concepts in engineering, physics, renewable energy, electrical generation and other fields. It takes energy to power vehicles, but the same task may be performed by energy in different forms (gasoline, lithium-ion batteries, etc.), as designed by engineers to meet specific functional requirements.
Learning Objectives
After this lesson, students should be able to:
- Explain the relationship between a moving object's kinetic energy and its mass and velocity.
- Identify different forms of kinetic and potential energy.
- Relate daily life experiences to different types of energy.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS3-5. Construct, use, and present arguments to support the claim that when the kinetic energy of an object changes, energy is transferred to or from the object. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Science knowledge is based upon logical and conceptual connections between evidence and explanations. Alignment agreement: Use mathematical representations to describe and/or support scientific conclusions and design solutions.Alignment agreement: | When the motion energy of an object changes, there is inevitably some other change in energy at the same time. Alignment agreement: | Energy may take different forms (e.g. energy in fields, thermal energy, energy of motion). Alignment agreement: |
Common Core State Standards - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Recognize and represent proportional relationships between quantities.
(Grade
7)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Energy is the capacity to do work.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
California - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Recognize and represent proportional relationships between quantities.
(Grade
7)
More Details
Do you agree with this alignment?
California - Science
-
Construct, use, and present arguments to support the claim that when the kinetic energy of an object changes, energy is transferred to or from the object.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/ucd_energy_lesson02] to print or download.Introduction/Motivation
In the previous lesson, we learned that energy is the ability to make things happen. Energy is in use everywhere and comes in many different forms. It can be stored and then employed to do things for us. In fact, all types of human activities require the input of energy. Gasoline is necessary to power automobiles, ships and airplanes so that we can travel long distances. Electricity powers light bulbs so we can continue to study, work and have fun after dark. Fireplaces, electrical heaters and gas furnaces provide warm indoor air so we can survive when the weather is freezing. And food, grown with the energy from the sun, is our fundamental source of energy to support our lives. Everywhere you look, in all sorts of different forms, energy is present and important in our daily lives.
(Continue by showing the presentation and delivering the content in the Lesson Background section.)
Lesson Background and Concepts for Teachers
Teach the lesson using the 15-slide PowerPoint file, Kinetic and Potential Presentation, along with the notes included below each slide. The presentation is animated, so clicking brings up the next image, text or slide. The presentation contains review questions and activities that ask students to use their new energy knowledge. The associated activity, Making Moon Craters, also provides opportunities to discuss and review key concepts with a fun and hands-on demonstration.
(Slide 2) As a class, verify students' understanding of concepts learned in the previous lesson by asking them to define the following terms: energy, kinetic energy, potential energy and energy transfer.
(Slide 3) Knowing that kinetic energy is energy of motion, ask students: What does kinetic energy depend on? Listen to student responses.
(Slide 4) Show the images of the blue car and truck. Now ask students, which one has more kinetic energy? Why? Given that both vehicles are traveling at the same speed (Listen to student responses, guiding them to say that the truck does). Ask students what this tells us kinetic energy depends on? (Answer: the mass of an object)
(Slide 5) Now show students the images of the blue car and the racing car. In this case, they both have the same mass. Ask students, which one has more kinetic energy? Why? Listen to student responses.
Ask students what this tells us kinetic energy also depends on? (Answer: the velocity of an object)
(Slide 6) Show students the equation for the kinetic energy of a non-rotating solid object: KE = (1/2) x mass x velocity^2. The two quantities, mass and velocity, thus define the kinetic energy of an object. For students to get a better sense of how KE varies with mass and velocity, it is helpful to graph the relationship on the classroom board or show students a simulation to demonstrate the relationship, such as The Ramp from PHET Interactive Simulations at https://phet.colorado.edu/en/simulation/the-ramp.
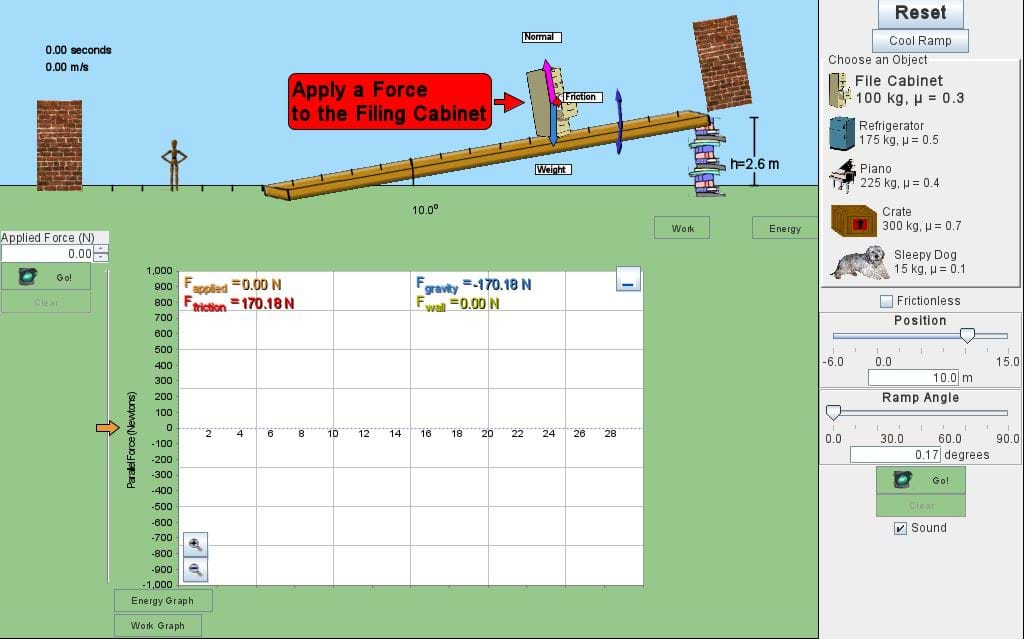
(Slide 7) Now revisiting the car and truck example, ask students how much more kinetic energy does the truck have compared to the car? (Answer: 2 times)
(Slide 8) Ask students, how much more kinetic energy does the racing car have compared to the blue car? (Answer: 4 times)
(Slides 9) Tell students, we know that potential energy is stored energy, but what ways can it be stored?
(Slide 10) Show students the photos of the roller coaster, downhill mountain biking and an elevated water tank. Ask students, where is the potential energy stored for these examples? (Answer: In the object’s height). Does anyone know what this potential energy is called? (Answer: Gravitational energy). What other examples of gravitational energy can you think of?
(Slide 11) Show students the images of coiled metal springs, rubber bands and a mattress. Ask students, where is the potential energy stored? (Answer: In the stretching or compression of the object). Does anyone know what this potential energy is called? (Answer: Elastic energy) What other examples of elastic energy can you think of?
(Slide 12) Show students the photo of the tea kettle on a stove burner. Ask students, where is the potential energy stored? (Answer: In the temperature of the object). Does anyone know what this potential energy is called? (Answer: Thermal energy) What other examples of thermal energy can you think of?
(Slide 13) Show students the images of the wood and little girl eating a peach. Ask students what type of potential energy is stored? (Answer: Chemical energy)
One of the most important concepts about energy is that energy can be neither created nor destroyed; it can only be transferred, or converted, from one form to another. For example, the chemical energy in wood (biomass) can be transferred to different forms of energy (light, heat, sound). Also, you may be active during recess or during gym because you eat meals to maintain your physical strength; in this case, the chemical energy stored in food (in the form of chemical bonds) is converted into kinetic energy (perhaps running around). Can you think of some more examples of energy conversion from one form to another?

(Slide 14) Make the final point that even though we've talked about many forms of energy, they are all different forms of the same thing. Remind students that energy can be converted from one form to another.
(Slide 15) Using the writing prompt on this slide (and description in the Assessment section), assign students to write about three fictional superpowers, and then read them to the class. Make sure students communicate using the energy terms learned in the lesson. Then conclude by administering the post-quiz.
Associated Activities
- Making Moon Craters - During a class demonstration, a weighted plastic egg is dropped into a tray of flour from different heights as a way to model asteroids hitting the moon's surface. By watching the potential-to-kinetic energy transfer and measuring the resulting impact craters, students directly see the effect that the height and mass of an object has on the overall energy of that object. Students learn the kinetic and potential energy equations, make predictions, and collect and graph data.
Vocabulary/Definitions
chemical energy: Energy that is stored in the chemical bonds of molecules. This energy is released during chemical reactions.
elastic energy: Energy that is stored in the stretching or compression of objects.
energy: The ability to make things happen. More advanced definition: The ability to do work.
gravitational energy: Energy that is stored in the height of objects. Gravity is the force that pulls things down to Earth. The higher an object, the more gravitational energy it has. Often, gravitational energy (a form of potential energy) is converted to kinetic energy to make things move fast.
kinetic energy: The energy of moving objects. Anything in motion has kinetic energy. The faster an object moves, the more kinetic energy it has.
potential energy: Energy that is stored and can be used when needed. Energy can be stored in chemicals (food, batteries), height (gravitational), elastic stretching, etc.
thermal energy: Energy that is stored in an object's temperature. Heat.
Assessment
Pre-Lesson Assessment
Definition Review: To verify students' understanding of concepts learned in the previous lesson, ask them to define the terms listed on slide 2 of the Kinetic and Potential Presentation: energy, kinetic energy, potential energy and energy transfer.
Post-Introduction Assessment
Discussion Questions: Use class discussions and student writing assignments to evaluate student knowledge. Throughout the Kinetic and Potential Presentation are many opportunities for quick assessments of understanding. For example, at slides 7 and 8 have students answer to fill in the blanks (before the answers are shown) to demonstrate their understanding.
Lesson Summary Assessment
Superpower Writing: Use the writing prompt on slide 15 to assess learning from this lesson: You are a superhero! You have three energy superpowers! Write a paragraph explaining: What are your superpowers? How do you use them? What kind of energy do they use? Require students to use the energy terminology learned in the lesson (and provided in a word bank on the slide). After students are done, give them time to share their answers with the class.
Post-Quiz: In addition, administer the Kinetic and Potential Post-Quiz to assess students' understanding of the relationship between mass, velocity and the kinetic energy of an object, as well as their ability to identify examples of various types of energy.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn more about the concept of energy conversion, and how energy transfers from one form, place or object to another. They learn that energy transfers can take the form of force, electricity, light, heat and sound and are never without some energy "loss" during the process. Two real-world ...

Students are introduced to the definition of energy and the concepts of kinetic energy, potential energy, and energy transfer. This lesson is a broad overview of concepts that are taught in more detail in subsequent lessons and activities in this curricular unit.

Students are introduced to the concepts of force, inertia and Newton's first law of motion: objects at rest stay at rest and objects in motion stay in motion unless acted upon by an unbalanced force. Students learn the difference between speed, velocity and acceleration, and come to see that the cha...

High school students learn how engineers mathematically design roller coaster paths using the approach that a curved path can be approximated by a sequence of many short inclines. They apply basic calculus and the work-energy theorem for non-conservative forces to quantify the friction along a curve...
Copyright
© 2014 by Regents of the University of Colorado; original © 2013 University of California DavisContributors
Eric Anderson, Jeff Kessler, Irene ZhaoSupporting Program
RESOURCE GK-12 Program, College of Engineering, University of California DavisAcknowledgements
The contents of this digital library curriculum were developed by the Renewable Energy Systems Opportunity for Unified Research Collaboration and Education (RESOURCE) project in the College of Engineering under National Science Foundation GK-12 grant no. DGE 0948021. However, these contents do not necessarily represent the policies of the National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: August 30, 2021






User Comments & Tips