Quick Look
Grade Level: 11 (10-12)
Time Required: 1 hour
Expendable Cost/Group: US $15.00
Group Size: 2
Activity Dependency:
Subject Areas: Algebra, Biology, Chemistry
NGSS Performance Expectations:

| HS-PS4-3 |

Summary
To become familiar with the transfer of energy in the form of quantum, students perform flame tests, which is one way chemical engineers identify elements—by observing the color emitted when placed in a flame. After calculating and then preparing specific molarity solutions of strontium chloride, copper II chloride and potassium chloride (good practice!), students observe the distinct colors each solution produces when placed in a flame, determine the visible light wavelength, and apply that data to identify the metal in a mystery solution. They also calculate the frequency of energy for the solutions.Engineering Connection
Before beginning to design a solution to a problem, engineers must research the concepts involved. As part of their research, chemical engineers use the flame test to identify an element based on the color it emits when placed in a flame—a low-tech and reliable method of identification. This unit's challenge question includes aspects of environmental engineering (effect of pollution on the Earth's ozone layer and consequences to public health) and biomedical engineering (use of nanoparticles in medical technologies).
Learning Objectives
After this activity, students should be able to:
- Explain that energy received by electrons to move them to an excited state is quantized.
- Explain that some wavelengths of light are in the visible light range while others are not, but nonetheless can cause chemical reactions.
- Calculate the number of grams of three ionic salts needed to make the appropriate molarity of solution.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS4-3. Evaluate the claims, evidence, and reasoning behind the idea that electromagnetic radiation can be described either by a wave model or a particle model, and that for some situations one model is more useful than the other. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Create or revise a simulation of a phenomenon, designed device, process, or system. Alignment agreement: Use mathematical representations of phenomena or design solutions to describe and/or support claims and/or explanations.Alignment agreement: | [From the 3–5 grade band endpoints] Waves can add or cancel one another as they cross, depending on their relative phase (i.e., relative position of peaks and troughs of the waves), but they emerge unaffected by each other. (Boundary: The discussion at this grade level is qualitative only; it can be based on the fact that two different sounds can pass a location in different directions without getting mixed up.) Alignment agreement: Electromagnetic radiation (e.g., radio, microwaves, light) can be modeled as a wave of changing electric and magnetic fields or as particles called photons. The wave model is useful for explaining many features of electromagnetic radiation, and the particle model explains other features.Alignment agreement: | Models (e.g., physical, mathematical, computer models) can be used to simulate systems and interactions—including energy, matter, and information flows—within and between systems at different scales. Alignment agreement: |
Common Core State Standards - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Reason quantitatively and use units to solve problems.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Energy can be grouped into major forms: thermal, radiant, electrical, mechanical, chemical, nuclear, and others.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Tennessee - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Reason quantitatively and use units to solve problems.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Tennessee - Science
-
Design and conduct scientific investigations to explore new phenomena, verify previous results, test how well a theory predicts, and compare opposing theories.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Use appropriate tools and technology to collect precise and accurate data.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Apply qualitative and quantitative measures to analyze data and draw conclusions that are free of bias.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Calculate the wavelength, frequency and energy of a photon of electromagnetic radiation.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Understand the mathematical principles associated with the science of chemistry.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Utilize appropriate mathematical equations and processes to solve chemistry problems.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- calculator
- graduated cylinder
- 3 400-ml beakers
- 6 wooden splints
- 3 plastic spoons
- electronic balance
- 3 pieces of paper (to put on the balance for massing the chemicals)
- matches
- Bunsen burner
- masking tape and marker, to label beakers
- Flame Test Worksheet, one per student
- safety goggles, one per student
- lab coat, one per student
- pair of rubber gloves, one per student
To share with the entire class:
- 100 grams strontium chloride, such as http://www.sciencecompany.com/Strontium-Chloride-100g-P15973.aspx
- 100 grams copper II chloride, also known as cupric chloride, such as http://www.sciencecompany.com/Cupric-Chloride-100g-P6404.aspx
- 100 grams potassium chloride, such as http://www.sciencecompany.com/Potassium-Chloride-100g-P15972.aspx
- distilled water, 1 gallon (~4 liters)
- 400-ml beaker for the unknown solution
- masking tape and marker, to label beaker
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/van_nanoparticles_lesson01_activity1] to print or download.Pre-Req Knowledge
Information about the electromagnetic spectrum and the Grand Challenge for this curricular unit are provided in the associated lesson, Electromagnetic Radiation, which should be taught previous to this activity.
Introduction/Motivation
Today we will look closer at the electromagnetic spectrum and how radiant energy in the form of heat causes a jump from an electron's ground state to an excited state. Then, we will build on this information to study how the energy of ultraviolet radiation can cause cancer in humans and how that same kind of energy can, in turn, be used to treat cancer. We will also practice the important skill of making solutions of the correct molarity. Remember, engineers cannot begin designing solutions before researching the concepts surrounding a problem, which is an early stage of the engineering design process. So, in this activity we will be researching.
By placing atoms of a metal into a heat source, electrons can be induced to absorb energy and jump to excited energy states. Then, by emitting photons of light, they return to their ground states. The amount of energy in the photon determines its color; red for the lowest energy visible light, increasing energy through the rainbow of orange, yellow, green, blue, indigo and violet for the highest energy visible light. Photons outside the visible spectrum may also be emitted, but we cannot see them. Remember that ultraviolet follows violet as the spectrum increases in energy. Chemical engineers use this low-tech and very reliable flame test to identify an element based on the color it emits when placed in a flame.
The arrangement of electrons in an atom determines the sizes of the quantum jumps, and thus the energy and colors of photons emitted.
In this lab, we will prepare .2 M solutions of strontium chloride, copper II chloride and potassium chloride. We will test the solutions to identify the distinct color each metal ion produces when placed in a Bunsen burner flame. Then we will calculate the approximate frequency and energy of each wavelength of visible light. With what we learn from this collected data, we will identify the metal in a solution of unknown identity.
Procedure
Before the Activity
- Gather materials and make copies of the Flame Test Student Handout, one per student.
- Either print color copies (one per group) or display in the classroom the Figure 1 color scale to help students identify wavelengths based on flame color.
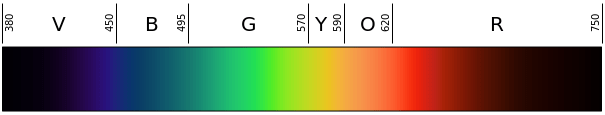
Figure 1. The visible light spectrum and corresponding colors-to-wavelengths in nanometers (nm). - Overnight, the night before the activity, soak all the wooden splints in distilled water. Just before the activity begins, pour out the water and rinse the splints with clean water.
- Choose one of the three chemicals to use for the "unknown solution." Prepare 100 ml of .2 M following the same instructions that students will follow in step 2, below. Label the beaker with masking tape and a marker as "unknown solution." Just before the activity, place wooden splints to soak in the unknown solution, one per group.
With the Students
- Hand out the worksheets and give students a few minutes to answer the pre-lab questions (also listed in the Assessment section, with answers), which includes their calculations of how much of three chemicals are needed to make three specific molarity solutions for the lab.
- Divide the class into groups of two or three students each. Have each group prepare the 100 ml each of .2 M strontium chloride, .2 M copper II chloride and .2 M potassium chloride, by doing the following:
- Place a piece of paper on the electronic balance and tare the balance. Add one of the chemicals (such as the strontium chloride) little by little using a clean spoon until the mass shown by the electronic balance is equal to the mass, in grams, of the chemical needed, calculated in question 4 of the worksheet pre-lab questions.
- Lift up the paper carefully with the appropriate quantity of the chemical and pour the chemical into a clean 400-ml beaker.
- Use the graduated cylinder to measure 100 ml of distilled water and add the water to the 400 ml beaker.
- Stir the beaker contents with the spoon used earlier to add the chemical to the electronic balance.
- Label the beaker by placing a piece of masking tape on the beaker and using a marker to write the name of the chemical used to make the solution.
- Discard the spoon and paper. Do not reuse them when making the other chemical solutions because they will contaminate the solutions.
- Repeat these steps to make solutions with the two remaining chemicals.
- Let two wooden splints soak in each solution for 10 minutes. (Suggested procedure: Have each group make one solution at a time, then add the wooden splint to the solution, and then make the next solution while the splint soaks.)
- Light the Bunsen burner with a lit match and adjust it to produce a moderately sized flame.
- Place the soaked end of the wooden splint into the hottest part of the Bunsen burner flame (the top of the inner cone).
- Record the observed flame color.
- Determine the approximate wavelength of the visible light corresponding with each observed flame color. Have students refer to Figure 1 to identify the wavelength based on the flame color. Remind students that the unit of length for wavelengths is nanometers (nm).
- Place a wooden splint soaked with the unknown solution in the Bunsen burner flame. Record the color and identify the metal ion in the solution, based on previous data collection results.
- Have students write a lab report that includes the five criteria listed on the worksheet, and explained in the Assessments section with answers).
Assessment
Pre-Activity Assessment
Pre-Lab Questions: Have students individually answer the following questions, which are provided on the Flame Test Worksheet. Review their worksheet answers to gauge their comprehension.
- List the electromagnetic spectrum from lowest to highest energy. (Answer: Radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, x-rays, gamma rays.)
- Of visible light, what color is lowest in energy? What is highest? (Answer: Lowest energy of visible light is red; highest energy of visible light is violet.)
- List all the cations and anions with charges present in this lab. (Answer: Anion is Cl-; cations are Sr2+, Cu2+, K+.)
- Perform the appropriate calculations for preparation of the three solutions: 100 ml of .2 M strontium chloride, 100 ml of .2 M copper II chloride and 100 ml of .2 M potassium chloride. (Answer: In order to prepare the solutions, students must determine how many grams of each compound must be added to 100 ml of distilled water to create the appropriate molarity solution. Refer to Figure 2 for the answer.)
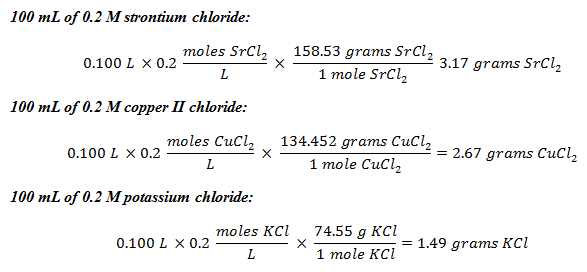
Figure 2. The molarity calculations for making the experimental solutions.
Activity Embedded Assessment
Experiment Conclusions: As directed in the Flame Test Worksheet, have students write typed lab reports that include the following information. If students do not finish within the class period, have them complete the assignment as homework to submit the following class period. Review their answers to assess their comprehension.
- Is it the metal or the non-metal that produces the flame test color? What brought you to this conclusion? (Answer: Flame tests show the color of the metal, or the positive ion [cation] in the chemical solution. Expect students to be able to come to this realization because the three chemicals had different metal atoms, but they all had the same non-metal anion, chloride.)
- The identity of the unknown (it is one of the metals you tested) (The answer depends on which chemical the teacher chose to use for the "unknown solution.")
- Show the calculations for the preparation of the three solutions. (Calculations shown in Figure 2.)
- Calculate the approximate frequency of energy given off by the emitting element in each of the three solutions. (Answers: Students should first estimate the wavelength of light produced when each solution burns, based on comparing the color of the flame to an image of the visible light spectrum, such as Figure 1. Images of flames produced using some of the chemicals in this activity, as well as others, are shown at: https://en.wikipedia.org/wiki/Colored_fire. Expect students to approximate the following wavelengths: for strontium chloride: around 700 nm; for copper II chloride: around 515 nm; and potassium chloride: around 450 nm. Then they should use the equation taught in lesson 3: ν = c/λ, where c = 3.0 x 108 m/s. The approximate answers for frequencies of energy are as follows: for strontium chloride: ν = 4.3 x 1014 hertz (Hz) = 430 terahertz (THz); for copper II chloride: 5.8 x 1014 Hz = 580 THz; for potassium chloride: 6.7 x 1014 Hz = 670 THz.)
- How does the flame test investigation relate to the engineering design process? (Answer: The flame test is part of the research phase of the engineering design process, which is important so that engineers understand all of the science and math involved in the problem they are trying to solve.)
Post-Activity Assessment
Going Further: As directed in the Flame Test Worksheet, have students answer the following problem on a separate sheet of paper: The energy you observed was given off in the visible light range. Where is ultraviolet light on the electromagnetic spectrum? Does it still contain energy even though it is not in the visible light range? Support your answer. (Answer: Ultraviolet light is next to violet visible light on the electromagnetic spectrum. This means that it has a smaller wavelength than violet light, a higher frequency, and thus contains more energy than violet light, as well as all other colors of visible light.)
Safety Issues
The lab work in this activity requires the use of Bunsen burners, and thus open flames. School and class policies vary on the use of open flames. Adjust the procedures accordingly, for example, if only adults are permitted to work with open flames. Some of the chemicals can cause skin and eye irritation, and are hazardous if ingested. Have students wear safety goggles, lab coats and gloves while conducting the activity.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Through two classroom demos, students are introduced to the basic properties of lasers through various mediums. Students will gain an understanding of how light can be absorbed and transmitted by different mediums.

Through an introduction to the design of lighting systems and the electromagnetic spectrum, students learn about the concept of daylighting as well as two types of light bulbs (lamps) often used in energy-efficient lighting design. Students learn how the application of something as simple, and free,...
Copyright
© 2013 by Regents of the University of Colorado; original © 2010 Vanderbilt UniversityContributors
Michelle Bell, Amber SpolarichSupporting Program
VU Bioengineering RET Program, School of Engineering, Vanderbilt UniversityAcknowledgements
The contents of this digital library curriculum were developed under National Science Foundation RET grant nos. 0338092 and 0742871. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: March 8, 2021




User Comments & Tips