Summary
Students are presented with a hypothetical scenario that delivers the unit's Grand Challenge Question: To apply an understanding of nanoparticles to treat, detect and protect against skin cancer. Towards finding a solution, they begin the research phase by investigating the first research question: What is electromagnetic energy? Students learn about the electromagnetic spectrum, ultraviolet radiation (including UVA, UVB and UVC rays), photon energy, the relationship between wave frequency and energy (c = λν), as well as about the Earth's ozone-layer protection and that nanoparticles are being used for medical applications. The lecture material also includes information on photo energy and the dual particle/wave model of light. Students complete a problem set to calculate frequency and energy.Engineering Connection
As the first step in the engineering design process, engineers must fully understand the problem. To do this, they research all relevant science and math concepts, as well as all previous and current engineering designs related to the subject. As students tackle the challenge question, they learn background information on the science of ultraviolet light. They also learn about the impact of human-generated pollution on the ozone layer and its connection to public health through increased incidences of skin cancer—an environmental engineering connection. In addition, they learn that biomedical engineers are researching ways to use nanoparticles to diagnose and treat cancer because they are good imaging agents.
Learning Objectives
After this lesson, students should be able to:
- Calculate frequency or wavelength for electromagnetic radiation.
- Calculate the energy of a photon.
- List components of the electromagnetic spectrum in order of increasing energy.
- Explain that energy given to an electron is quantized.
- Explain what biomedical engineers study.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-1. Analyze a major global challenge to specify qualitative and quantitative criteria and constraints for solutions that account for societal needs and wants. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Analyze complex real-world problems by specifying criteria and constraints for successful solutions. Alignment agreement: | Criteria and constraints also include satisfying any requirements set by society, such as taking issues of risk mitigation into account, and they should be quantified to the extent possible and stated in such a way that one can tell if a given design meets them. Alignment agreement: Humanity faces major global challenges today, such as the need for supplies of clean water and food or for energy sources that minimize pollution, which can be addressed through engineering. These global challenges also may have manifestations in local communities.Alignment agreement: | New technologies can have deep impacts on society and the environment, including some that were not anticipated. Analysis of costs and benefits is a critical aspect of decisions about technology. Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-2. Design a solution to a complex real-world problem by breaking it down into smaller, more manageable problems that can be solved through engineering. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Design a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | Criteria may need to be broken down into simpler ones that can be approached systematically, and decisions about the priority of certain criteria over others (trade-offs) may be needed. Alignment agreement: | |
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS4-3. Evaluate the claims, evidence, and reasoning behind the idea that electromagnetic radiation can be described either by a wave model or a particle model, and that for some situations one model is more useful than the other. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Evaluate the claims, evidence, and reasoning behind currently accepted explanations or solutions to determine the merits of arguments. Alignment agreement: A scientific theory is a substantiated explanation of some aspect of the natural world, based on a body of facts that have been repeatedly confirmed through observation and experiment and the science community validates each theory before it is accepted. If new evidence is discovered that the theory does not accommodate, the theory is generally modified in light of this new evidence.Alignment agreement: | [From the 3–5 grade band endpoints] Waves can add or cancel one another as they cross, depending on their relative phase (i.e., relative position of peaks and troughs of the waves), but they emerge unaffected by each other. (Boundary: The discussion at this grade level is qualitative only; it can be based on the fact that two different sounds can pass a location in different directions without getting mixed up.) Alignment agreement: Electromagnetic radiation (e.g., radio, microwaves, light) can be modeled as a wave of changing electric and magnetic fields or as particles called photons. The wave model is useful for explaining many features of electromagnetic radiation, and the particle model explains other features.Alignment agreement: | Models (e.g., physical, mathematical, computer models) can be used to simulate systems and interactions—including energy, matter, and information flows—within and between systems at different scales. Alignment agreement: |
Common Core State Standards - Math
-
Solve linear equations and inequalities in one variable, including equations with coefficients represented by letters.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Medical technologies include prevention and rehabilitation, vaccines and pharmaceuticals, medical and surgical procedures, genetic engineering, and the systems within which health is protected and maintained.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Illustrate principles, elements, and factors of design.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Tennessee - Science
-
Calculate the wavelength, frequency and energy of a photon of electromagnetic radiation.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Understand the mathematical principles associated with the science of chemistry.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Utilize appropriate mathematical equations and processes to solve chemistry problems.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Describe the dynamic interplay among science, technology, and engineering within living, earth-space, and physical systems.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/van_nanoparticles_lesson01] to print or download.Introduction/Motivation
You have just been given the following grand challenge: "Congratulations! You have just won the Nobel Prize in chemistry for your work with nanoparticles! You have now decided to fulfill your lifelong dream of learning how to surf by using your 1.4 million dollar monetary prize to move to Einstein Cove, Australia. However, upon researching your move, you come across the ultraviolet index information for Australia. What does this mean in terms of your potential skin cancer risk as a surfing enthusiast in Einstein Cove? How could you use your expertise in nanoparticles to treat, detect and protect against skin cancer?"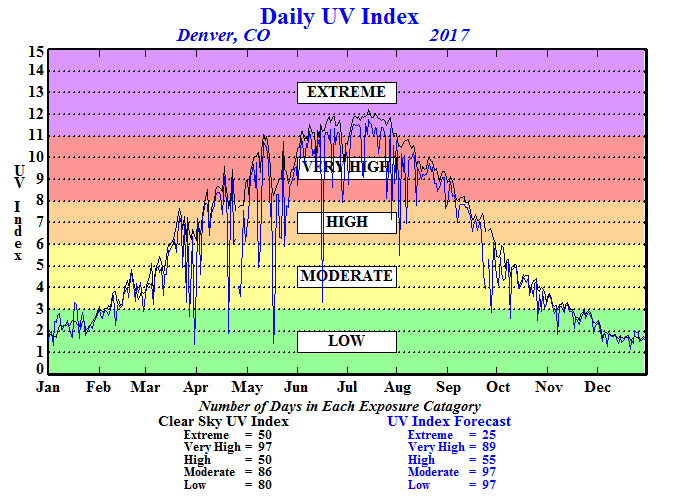
Biomedical engineers are increasingly looking into nanoparticles to find ways to diagnosis and treat skin cancer because nanoparticles are particularly good imaging agents. What are nanoparticles? And how could they be used to treat, detect and protect against skin cancer? What exactly is skin cancer? And what is the cause? These are just a few of the questions we investigate during the next few days in our lesson on using nanoparticles to detect, treat and protect against skin cancer.
Lesson Background and Concepts for Teachers
Legacy Cycle Steps for this Lesson
(Generate Ideas) Ask students the following research question: What is electromagnetic radiation? In class, have students write journal entries to answer the following questions:
- What are your initial ideas about how this question can be answered?
- What do you already know about UV light and nanoparticles?
(Multiple Perspectives) As a class, have students share ideas from their journals. Record their ideas on the board.
(Research and Revise) Deliver to students the lecture information on the electromagnetic spectrum, ultraviolet radiation (including UVC, UVB, and UVA rays), photon energy, and the relationship between, frequency, wavelength and energy.
(Test Your Mettle) Assign students to complete the Energy Homework questions and problems as homework or classwork.
(Go Public) Upon returning to class or completing the problem set, have students work the problems on the classroom board and discuss the answers if all students are not in agreement.
Following the lesson, students can conduct the hands-on Flame Test: Red, Green, Blue, Violet? activity to expand their understanding by gathering data that helps them identify the chemical makeup of a "mystery solution" based on the visible color the solution burns.
Lecture Information
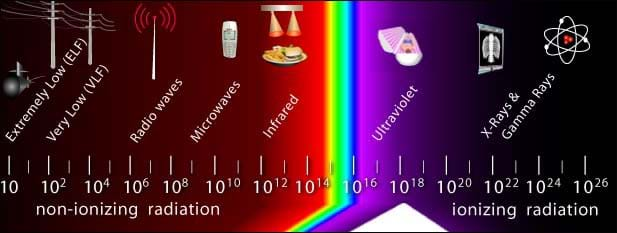
Ultraviolet light is a form of electromagnetic radiation. Electromagnetic radiation is a form of radiant energy that exhibits wave-like behavior and travels through space at the speed of light in a vacuum. The entire range of frequencies and wavelengths that make up all forms of electromagnetic radiation, including radio waves, gamma rays and ultraviolet waves is called the electromagnetic spectrum.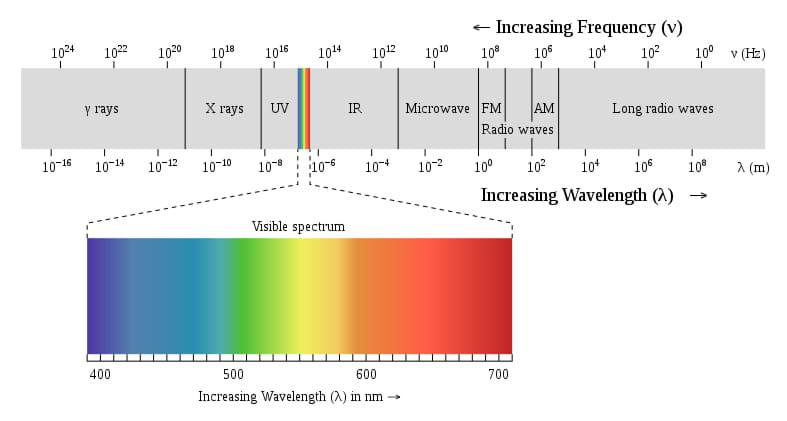
The relationship between wave frequency, wavelength and energy is described by the equation c = λν, where c = wave speed. Wave speed in a vacuum is 3.00 108 m/s. Wavelength is the shortest distance between points where the wave pattern repeats itself, such as from crest to crest or trough to trough and is represented by the symbol λ (lambda). Wave frequency is the number of complete oscillations that a wave makes each second; it is measured in hertz and is represented by the symbol ν. Thus, the speed of the wave is equal to frequency wavelength of the wave.
In 1900, Max Planck, a German physicist, postulated that energy is quantized and therefore must occur in discrete units of size, hν; thus, ΔE = hν. These units were called quanta of energy. Albert Einstein furthered this postulate through his Nobel Prize-winning analysis of the photoelectric effect. Einstein stated that electromagnetic radiation is also quantized. These discrete packets of electromagnetic radiation were called photons. Energy of a photon of electromagnetic energy is given by Ephoton = hν.
Combining the two equations, c = λν and Ephoton = hν, we see that Ephoton = hc/λ.
Through innovations of the combined work of Arthur Crompton, Planck and Einstein, it became evident that electromagnetic radiation has both wavelike and particle-like properties; this is now referred to as the dual nature of light. Further, Louis DeBroglie proposed that the same must also be true for matter. DeBroglie postulated that one could calculate the wavelength of a particle such as an electron given the mass and speed of the particle, through the equation m = h/λc.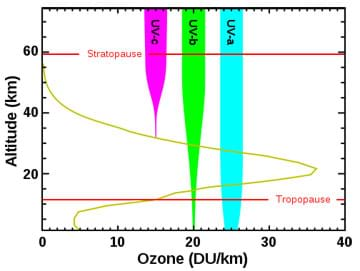
The sun emits three forms of ultraviolet radiation:
- UVA, with a wavelength range of 315-390 nanometers
- UVB, with a wavelength range of 280-315 nanometers
- UVC, with a wavelength range of 100-280 nanometers
A large percentage—98%—of UV radiation is blocked by the ozone layer in regions with sufficient ozone. All UVC and most UVB rays are stopped by the ozone layer naturally. In turn, 90% of the UV radiation that reaches the Earth's surface is UVA. Both UVA and UVB can cause significant health risks (which will be investigated in the next lesson). Before designing any protective products, engineers must first understand the nature of ultraviolet light to understand how these rays cause skin and cancer.
Associated Activities
- Flame Test: Red, Green, Blue, Violet? - Students perform a flame test, gathering data that helps them identify the chemical makeup of a "mystery solution" based on the visible color the solution burns. This activity gives students practice in preparing specific molarity solutions and helps them make connections between colors, light wavelengths, and energy frequencies, which are dependent upon the molecules in the solutions.
Vocabulary/Definitions
electromagnetic radiation: Radiant energy that exhibits wavelike behavior and travels through space at the speed of light in a vacuum.
electromagnetic spectrum: The entire range of frequencies and wavelengths that make up all forms of electromagnetic radiation, including radio waves and gamma rays.
emission spectrum: A plot of the intensity of light emitted from a hot body over a range of frequencies.
energy: The capacity to do work or cause the flow of heat.
excited state: The state of an atom with excess energy.
frequency: The number of complete oscillations that a wave makes each second; measured in hertz.
ground state: The lowest possible energy state of an atom.
photoelectric effect: The emission of electrons by certain metals that is produced when they are exposed to electromagnetic radiation.
photon: A discrete, quantized bundle of radiation that travels at the speed of light, has zero mass, and has energy and momentum; a particle of electromagnetic radiation.
Planck constant: 6.626 X 10 to the power of negative 34 J S
quantized: Occurring in discrete units of size, in the case of radiant hv.
quantum: One quantized packet of energy.
wavelength: The shortest distance between points where the wave pattern repeats itself, such as from crest to crest or trough to trough.
Assessment
Pre-Lesson Assessment
Review: Review with students the concepts about waves, wave properties and vocabulary, such as wavelength, frequency, amplitude, crest and trough. Ask them to name as many types of electromagnetic radiation as possible. (Example answers: Visible light, ultraviolet, infrared, microwaves, radio waves and gamma rays.) Show them a chart of the electromagnetic spectrum.
Post-Introduction Assessment
Research Challenge: Pose this research question to students: What is electromagnetic radiation? Then have students write journal entries to answer the following questions. As students share their answers with the class, write their ideas on the classroom board and use their answers to gauge their comprehension.
- What are your initial ideas about how this question can be answered?
- What do you already know about UV light and nanoparticles?
Lesson Summary Assessment
Brainstorming: Ask students to brainstorm in small groups the connection between electromagnetic radiation and the unit's Grand Challenge Question, as applies to skin cancer, including the potential risks and the use of nanoparticles to protect against skin cancer. Have them record their ideas about how what they learned in the lesson's lecture information could be useful in addressing the Grand Challenge Question.
Homework
Energy Homework: At lesson end, assign students to complete the 10-question Energy Homework, which requires them to use the equations and other information presented in the lesson to answer the questions and complete the problems. Refer to the Energy Homework Answer Key. Have students work the problems on the classroom board and discuss the answers if all students are not in agreement. Review students' individual answers to evaluate their depth of comprehension.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

After seeing ultraviolet-sensitive beads change color and learning how they work, students learn about skin anatomy and the effects of ultraviolet radiation on human skin, pollution's damaging effect on the ozone layer that can lead to increases in skin cancer, the UV index, types of skin cancer, AB...

Students apply the knowledge gained from the previous lessons and activities in this unit to write draft grant proposals to the U.S. National Institutes of Health outlining their ideas for proposed research using nanoparticles to protect against, detect or treat skin cancer. Through this exercise, s...

Students learn the basics of the electromagnetic spectrum and how various types of electromagnetic waves are related in terms of wavelength and energy. In addition, they are introduced to the various types of waves that make up the electromagnetic spectrum including, radio waves, ultraviolet waves, ...

This unit on nanoparticles engages students with a hypothetical Grand Challenge Question that asks about the skin cancer risk for someone living in Australia, given the local UV index and the condition of the region's ozone layer. Through three lessons, students learn about the science of electromag...
References
Zitzewitz, Paul W., et al. Physics: Principles and Problems. Columbus, OH: McGraw Hill, 2009.
Zumdahl, Steven S. and Zumdahl, Susan A. Chemistry. Seventh edition. Boston, MA: Houghton Miller Company, 2007.
Copyright
© 2013 by Regents of the University of Colorado; original © 2010 Vanderbilt UniversityContributors
Michelle Bell, Amber SpolarichSupporting Program
VU Bioengineering RET Program, School of Engineering, Vanderbilt UniversityAcknowledgements
The contents of this digital library curriculum were developed under National Science Foundation RET grant nos. 0338092 and 0742871. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: September 16, 2020







User Comments & Tips