Quick Look
Grade Level: 10 (10-12)
Time Required: 3 hours
(two 90-minute blocks)
Expendable Cost/Group: US $0.07 The $3 expendable materials cost investment lasts for 45 groups. This activity also uses some non-expendable (reusable) lab equipment and a centrifuge; see the Materials List for details.
Group Size: 4
Activity Dependency: None
Subject Areas: Chemistry, Physical Science, Physics, Science and Technology

Summary
Students learn about the separation techniques of sedimentation and centrifugation and investigate whether blood is a homogeneous or a heterogeneous mixture. Working in groups as if they are biomedical researchers, they employ the scientific method and make observations about the known characteristics of urine, milk and blood. They probe further by analyzing research on the properties and fractionation modes of blood. As students learn about certain strange characteristics with the fractionation behavior of blood, they formulate hypotheses on the unique nature of blood. Using provided materials —olive oil, tomato juice and petroleum jelly—they design an experiment and construct a blood model. They test their hypotheses by conducting experiments on the blood model, and then propose theories for the nature of blood as a mixture—arriving at the theory of mixture dualism in blood—that blood is a complex mixture system. An activity-guiding handout and PowerPoint® presentation are provided for this student-directed, project-based activity.Engineering Connection
This activity establishes for students a connection to real-world applications of separation technology. All sorts of mixture separation processes form the backbone of pervasive everyday industries such as food processing, water treatment, dairy processing and medical blood handling, in which engineers are intricately involved. Learning about two separation techniques—sedimentation and centrifugation—gives students a foundation from which to understand the value of the separation of mixtures concept. In fact, some type of industrial mixture separation processing is key to the chemical, petroleum refining, aluminum, steel, metal casting, glass, and paper and pulp industries. Two examples: The simple lab technique of centrifugation has great commercial and societal value when applied at the industrial-level for materials production. The simple phenomenon of sedimentation of particles from fluid suspension is the basis for the common ESR blood test used to measure body inflammation. Understanding the complex structure and composition of bio-macromolecular mixture systems such as blood is important for biomolecular engineers as they create solutions to life science and health problems.
Learning Objectives
After this activity, students should be able to:
- Describe blood as a complex mixture system composed of several mixture subsystems.
- Explain how blood can separate in different ways under different conditions both inside and outside the body.
- Analyze why sedimentation and centrifugation are widely used in the separation of blood into its constituents.
- Show that the ESR clinical test is based on the sedimentation technique for blood separation.
- Show that most clinical blood analysis tests (other than ESR test) and the blood product industry use the centrifugation technique to separate blood into different components.
- Formulate a hypothesis on the behavior of blood as a mixture.
- Construct a blood model from everyday materials by applying the concepts of density, miscibility, solubility, etc.
- Experimentally demonstrate the sedimentation process in blood, the centrifugation of blood using a blood model, and the relationship between sedimentation and centrifugation.
- Theorize that blood exhibits mixture dualism.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
Develop a model based on evidence to illustrate the relationships between systems or between components of a system.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
Common Core State Standards - Math
-
Define appropriate quantities for the purpose of descriptive modeling.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Models are used to communicate and test design ideas and processes.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
-
Evaluate designs based on criteria, constraints, and standards.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
-
Modeling, testing, evaluating, and modifying are used to transform ideas into practical solutions.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Connect technological progress to the advancement of other areas of knowledge and vice versa.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Georgia - Math
-
Add, subtract, and multiply algebraic expressions.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Students will generate and interpret equivalent numeric and algebraic expressions.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Georgia - Science
-
Students will analyze anatomical structures in relationship to their physiological functions.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Students will analyze the physical, chemical, and biological properties of process systems as these relate to transportation, absorption and excretion, including the cardiovascular, respiratory, digestive, excretory and immune systems.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Relate the structure of the integumentary system to its functional role in protecting the body and maintaining homeostasis.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Students will analyze the nature of matter and its classifications.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Explain that further understanding of scientific problems relies on the design and execution of new experiments which may reinforce or weaken opposing explanations.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Students will investigate the properties of solutions.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Students will analyze the relationships between force, mass, gravity, and the motion of objects.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Students will use standard safety practices for all classroom laboratory and field investigations.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Students will demonstrate the computation and estimation skills necessary for analyzing data and developing reasonable scientific explanations.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- Self-Guided Learning Module Handout, one per student
- V8 beverage, 15 ml kept in a labeled clear container; V8 is a tomato and vegetable juice mixture
- 1% solution of petroleum jelly in olive oil, 15 ml kept in a labeled, clear container; follow the solution preparation instructions in the Procedure-Before the Activity section
- 15-ml graduated plastic centrifuge tube with cap, such as those available at https://www.amazon.com/s?k=15-ml+graduated+plastic+centrifuge+tube+with+cap&ref=nb_sb_noss
- 3 thin wooden coffee stirrer sticks
- 2 graduated centrifuge tubes
- plastic dropper
- lab aprons and goggles, for each student
- test tube holder
- (optional) 2 trays
To share with the entire class:
- computer with Internet access and a projector to show the class the Heterogeneous vs. Homogeneous Mixtures Presentation, a PowerPoint® file, and four short online YouTube videos
- centrifuge; note: electrically operated lab model centrifuges are commonly available in U.S. high school chemistry labs and many other schools often have hand centrifuges affixed on the corners of lab work benches; the centrifuge tubes, which are the tapered test tubes, are indispensable accessories for the centrifuge; recommended sources to purchase centrifuges are Flinn Scientific Company, Carolina Biological Company, and Spectrum Chemical Manufacturing Corporation
- supplies to prepare a 1% solution of petroleum jelly in olive oil: glass beaker, digital scale, plastic spatula or spoon, 1 g Vaseline petroleum jelly, graduated cylinder, 100 ml olive oil, glass stirring rod
- paper towels, a few cut into long thin pieces for clean-up of lab and centrifuge parts
- water, sink, bowl of soapy water, test tube brushes, paper towels, for lab equipment clean-up
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/gat_mixture_activity1] to print or download.Pre-Req Knowledge
Students should be familiar with the following topics:
- the distinction between mixtures and compounds
- gravity and gravitational pull
- centripetal force
- density
- blood cells, plasma and their functions
- types of mixtures and mixture separation techniques
- lab safety protocols
Introduction/Motivation
For doctors, biochemists, medical researchers and forensic scientists, a drop of blood is an ocean of information. Investigating blood in detail is a way to obtain evidence for providing appropriate medical treatment or ascertaining the facts related to a crime or casualty. Blood is a material of great interest for bioengineers as well. Around the world, numerous bioengineering groups are actively involved in blood research. Marveled by the fluid mechanics and circulatory motion of blood propelled by the pumping of a tiny muscular bag called the heart, bioengineers are engrossed in applying engineering principles to unravel the complex phenomena in blood flow.
Blood is truly the most marvelous biological fluid, and is multifunctional and dynamic. Blood holds in it innumerable constituents that are relatively high in concentration. Yet blood perfectly holds them all uniformly, although it must constantly flow through an extensive pipe system of varying diameters, with different wall characteristics. Blood behaves perfectly like a single fluid, with each solute enjoying perfect freedom to offer its definite function. The various collective functions for blood constituents are also performed with ease! Obviously, blood is a mixture, but many questions arise as we think of blood's meticulous functioning:
- Given the crowding and close proximity of different solutes, how do the solutes separate out individually to perform their independent functions?
- Is blood a homogeneous mixture or a heterogeneous mixture?
- With so many uniquely different solutes, how are they all so competently managed?
- What is the role of fluid mechanics in the functional integrity of blood?
- What is the relationship between the pipe system of blood vessels and the blood?
- How do shear phenomena influence the behavior of blood?
- What sort of undesired events occur in blood flow?
All these questions are linked to the intriguing behavior of blood as a mixture. Gaining a thorough knowledge of the nature of blood as a mixture and its behavior as a flowing mixture is extremely useful in understanding the influences of various forces on blood, natural or inadvertent. We know that blood separates in different ways depending on what forces are acting upon it. Such knowledge is generated from bioengineering studies on blood that help us unravel the complexity associated with various blood phenomena including undesired fatal medical conditions such as thrombosis.
(Proceed to present to the class the 16-slide Heterogeneous vs. Homogeneous Mixtures Presentation, a PowerPoint® file. Note that the slides are "animated," so clicking the mouse or keyboard advances the next text, image or slide.)
Procedure
Overview
This student-directed, project-based activity provides students with a deeper understanding of liquid/solution-based mixtures and their everyday applications. During the activity, students encounter substances and situations of relevance in their daily lives, especially materials secreted/excreted in their own bodies or secretions from familiar animals, such as milk. For this purpose, this activity begins with the motivation strategy of students making a comparative analysis of urine, blood and milk.
Students proceed further in a self-regulated/guided learning process, throughout this activity, guided by a 16-page handout. Self-regulated learning calls for great teaching demands. Refer to the Teacher Notes & Tips for important suggestions in preparing for this activity's teaching requirements. Since the activity is presented in a project-based format with one task leading to another, it helps if transitions are well planned.
The lab part of this activity is designed for students to work in groups of four. All other tasks can also be done in teams of four, however, the group size for the other tasks is at the teacher's discretion since s/he knows best about the nature of individual students.
Schedule
Day 1: Conduct part 1 of the activity, from the bell-ringer quiz to interim summarization assessment, assigning the pre-lab planning as homework.
Day 2: Conduct part 2 of the activity, including the bell-ringer quiz, lab session, post-lab inquiry, proposing the theory of mixture dualism of blood, evaluating the relationship between sedimentation and centrifugation, and technical essay writing on different types of centrifugation as homework.
Teacher Background
Mixtures can have a liquid component. Most commonly known liquids are solvents as well. In a solvent, solutes can be dissolved, dispersed or suspended; this yields three kinds of solutions: true solutions, colloidal solutions and suspensions. Both true solutions and colloidal solutions are uniform in composition throughout the solution. Hence, they are homogeneous solutions. On the other hand, a suspension has an insoluble and separated-out solute, and therefore is a heterogeneous solution. The PowerPoint® presentation covers homogeneous and heterogeneous solutions. In addition, refer to several Internet resources on this topic listed in References section.
An activity of this nature (involving complex concepts) is sure to involve curious questions from students, especially with regard to states of matter and phase. Engage them in an intellectual discussion. Bring to focus that a phase of matter is characterized by the presence in a matter of moderately uniform chemical and physical properties. Phases are different from states of matter. Matter can exist in different phases yet be in the same state of matter. For example, mixtures can exist in multiple phases, such as an oil phase and an aqueous phase, etc. (This same explanation is also provided in the slide 7 notes.) For more information about states of matter, refer to the following resource: http://www.chem1.com/acad/webtext/states/states.html.
Expanded Engineering Connection
Mixture separation processes form the backbone of innumerable industries, which in turn require highly diverse and specialized technologies. For certain industries, separation is the mainline activity, such as the water, food processing, and dairy and blood components industries. The seven mega-scale "industries of the future" identify mixture separation as their chief industrial activities: chemical, petroleum refining, aluminum, steel, metal casting, glass, and paper and pulp industries. In addition, separation processes also present opportunities for wastewater treatment, waste reduction, and more efficient use of energy and raw materials.
This activity focuses on two separation techniques—sedimentation and centrifugation, which have many direct engineering connections. The direct industrial-level application of a simple laboratory technique of separation of mixtures, namely centrifugation, in the industrial-level production of materials has great commercial and societal value—such as in the blood industry. The simple phenomenon of sedimentation of particles from a fluidized suspension was directly exploited to develop a clinical blood analysis test, namely, the erythrocyte sedimentation rate (ESR) test—now a common blood test used to detect and monitor body inflammation.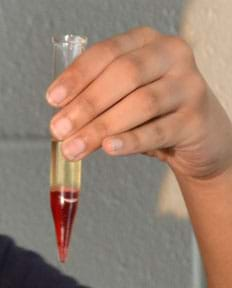
Biomolecular engineering is a special interdisciplinary branch of engineering focused on the purposeful manipulation of molecules of biological origin. Thus, the complex architecture and composition of bio-macromolecular mixture systems such as blood is important for engineers to understand. Bioengineers combine their understanding of natural biological mixture systems with their core knowledge of chemical and mechanical engineering to find solutions to issues and problems in the life sciences and healthcare sector.
Before the Activity
- Gather and organize materials for the lab.
- Make copies of the 16-page Self-Guided Learning Module Handout, one per student. If it is not possible to color print, arrange to pull up the colorful visuals on the projector for students to see.
- Print out the Self-Guided Learning Module Handout Answer Key, especially the Interim Summarization section so that you are prepared to grade student responses quickly.
- Create a 1% solution of petroleum jelly in olive oil by dissolving 1 gram of petroleum jelly thoroughly in 100 ml of olive oil. Detailed instructions: Place a clean dry beaker on a digital scale and tare it. With a clean plastic spatula or spoon, take a small quantity of petroleum jelly and find the mass. If it is above or below 1 g, remove or add some petroleum jelly until the scale reads 1 g. Remove the beaker from the scale. Measure 100 ml of olive oil in a graduated cylinder. Add the 100 ml of olive oil in small portions while you use a glass stirring rod to constantly mix and dissolve the petroleum jelly into the olive oil. It is more difficult to dissolve the petroleum jelly if you add all the 100 ml olive oil in bulk on top of the petroleum jelly.
- Decide on student groupings and the appropriate classroom seating arrangement.
- Become familiar with the essential information and troubleshooting information provided in the Teacher Notes & Tips, as well as the handout and its answer key.
- Become familiar with the bell-ringer quiz for each day, which is on pages 1 and 12 of the handout.
- Prior to class, view the four videos that students will watch and take the necessary notes from them. The video URLs are listed on the student handout and in the Additional Multimedia Support section.
- For Day 1, set up the computer and projector to show the Heterogeneous vs. Homogeneous Mixtures Presentation and four online video clips. Find all the video clips and, for some, set the required portion to commence. Having the videos ready to play on different browser tabs helps with time and classroom management.
- To prepare for the Day 2 experimental part of the activity, arrange the materials in an orderly manner with labels so students can execute the activity without issues. Keeping a tray below the V8 beverage as well as the oil-jelly blend helps to contain spills, keep work spaces clean and speed up cleaning.
- During the Day 2 lab session, make sure students wear aprons and goggles for lab safety.
With the Students: Day 1
- As students enter the class, have them pick up a handout and complete the pre-assessment bell-ringer quiz.
- Once students have finished the quiz, check the answers with them to clarify any misconceptions. If the prerequisite skills have been mastered by students, checking answers with them takes very little time.
- Present the Introduction/Motivation content to the class.
- Show students the slide presentation.
- Tell students that today they are acting as if they are biomedical researchers challenged to make models of blood and then conduct an experiment to discover the answer to the question: What type of mixture is blood? They will follow the scientific method and propose a theory for the mixture of blood. Explain to them that this is similar to what some scientists and engineers do for their jobs.
- Make sure that students understand that the term "blood" in this activity refers to human blood.
- Have students complete the Activation Strategy section of the handout, which includes Tasks 1 and 2 on pages 1-3 (takes 13-15 minutes). Facilitate the self-directed learning by moving around and monitoring their actions, and proactively supporting them. Expect most students to get correct answers for these tasks, however, review their answers quickly before students proceed to Task 3.
- Have students begin working on Task 3 in the Class Work-1 section of the handout starting on page 4.
- When students reach Task 3.2, play the video clips and tell students to take notes on their handouts. Play each video one more time if needed. To be efficient with time, start and end each video at the times specified on page 5 of the handout.
- Have students complete the rest of the Class Work-1 section, which includes Tasks 3.3 and 3.4. In Task 3.4, students each formulate a hypothesis about the nature of blood as a mixture.
- Have students work on the Class Work-2 section of the handout, which prepares them for the experimental design part of the activity on Day 2. The Class Work-2 section includes Tasks 4 through 7. In Task 5, students calculate the weighted average specific gravity of blood by multiplying the average specific gravity of each blood constituent (plasma, buffy coat and erythrocytes) by its percent by volume and summing the results. While students work on this section, circulate the room to answer any questions they may have.
- For homework, assign Task 8, the Pre-Lab Activity, on page 11 of the handout.
With the Students: Day 2 — Experimentation
- Have students complete the bell-ringer quiz on page 12 of the handout.
- Divide the class into groups of four students each.
- Before starting the experimental part, check for understanding and review safety issues:
- Make sure groups are clear on what they will be doing in the experimental part. Remind them to read the labels before they take or use any material.
- Review lab safety and have students put on aprons and goggles. Tell them that lab aprons and safety goggles are worn to protect themselves and their clothes in the event of spills, since even benign materials can stain. Goggles protect students' eyes in case of oil or V8 splashing. Refer to the suggested guidelines in the Safety Issues section.
- Review safety issues with the centrifuge, especially that it will only be operated by the teacher. Indicate the procedures: Groups provide the teacher with samples and the teacher spins the samples for them.
- After these preliminary tasks, direct students to assemble at the lab stations, put on the lab aprons and goggles, and begin their experimental investigations, guided by the handout.
- Advise students to use the very minimum of the materials for their experiments. Remind them of the reduce-reuse-recycle policy as the best practice method.
- For blood model construction, limit students to using only the materials provided. Tell them what materials are provided and how they are arranged for the group.
- Ask students to explain why these materials are provided to them to create blood models.
- Direct groups to conduct the lab as guided by the Task 9 instructions on the handout. Make sure students record their observations and inferences in the chart on page 12 of the handout.
- As groups conduct their lab experiments, circulate the room and monitor each group. Ask students to correlate their findings to the clinical test of erythrocyte sedimentation rate test (ESR test), and to the dynamic and static behavior of blood. (optional) Ask them to explore the rate of mixing on the consistency of the blood model.

Figure 1. Centrifuged blood model. - After the experimental part, and based on their experiments and acquired knowledge throughout this activity, have students complete Task 10 in which they develop theories for the mixture nature of blood. During the theory development, students may need a lot of support:
- Initiate an interactive discussion and check with each group to see whether or not they proved the hypotheses they proposed.
- Discuss how to propose a theory. Talk about postulates. Give an example, such as Dalton's Atomic Theory. Facilitate as students frame their own postulates.
- Help students by providing words like dualistic, dual, dualism, etc. Help them construct the postulates based on the experimental evidence.
- The postulates may be presented in any hierarchy; appreciate their efforts and motivate them to proceed successively through the theory construction.
- Have students complete Task 11, using the Internet for research to gain an understanding of how sedimentation and centrifugation are related, and compose a summary explanation with diagrams and cited references.
- For homework, assign Tasks 12 and 13, to research and write technical essays that 1) describe the different types of centrifugation techniques, including the principle behind each and special applications of each, and 2) summarize the value of the blood processing industry and its career paths.
- Conclude by conducting the remaining post-assessment activities described in the Assessment section.
Vocabulary/Definitions
anticoagulant: A substance that hinders coagulation.
blood clot: A mass of coagulated blood.
buffy coat: The very thin middle fraction of the centrifuged anticoagulated blood that contains most of the white blood cells and platelets.
centrifugation: The technique of using centrifugal force for accelerating the sedimentation of heterogeneous mixtures via a centrifuge machine.
coagulation: The process by which a solution or a colloidal solution turns into a semisolid mass.
colloidal solution: A solution (dispersed phase) in which solute particles of dimensions between approximately 1 nm and 1 μm are uniformly dispersed in a solvent (dispersion medium).
density: The mass of unit volume of a substance; unit: kg per meters squared
dispersed phase: Solute particles of dimensions between approximately 1 nm and 1 μm that are uniformly dispersed in a solvent (dispersion medium)
dispersion medium: The solvent in a colloidal solution.
erythrocyte sedimentation rate: A measure of the settling ability of red blood cells in a thin, tall, vertical tube of blood during a time of one hour; abbreviated as ESR.
heterogeneous mixture: A mixture of non-uniform composition that is easily separable into individual constituents by simple separation techniques.
homogeneous mixture: A mixture of uniform composition that is not easily separable into individual constituents by simple separation techniques, but can be separated by special separation techniques.
phase: A state of matter that is homogeneous in composition and has clearly defined boundaries.
pigment: A substance that imparts color to a material.
plasma: The fundamental liquid matrix of blood that holds the blood constituents.
platelet: A minute, non-nucleated, disk-like cytoplasmic body found in the blood plasma of mammals; also called blood platelet or thrombocyte.
red blood cell: A non-nucleated cell that is biconcave in shape; also called erythrocyte.
sedimentation: The process by which dispersed solute particles in a solution settle out of the solution and fall to the bottom of the container, and subsequently thicken.
serum: The liquid portion of clotted blood.
specific gravity: A measure of how denser a substance is with reference to a standard substance; the general reference substance for liquids and solutions is water at 4 °C, which has a unit density.
thrombosis: The presence in the artery or vein of a blood clot.
white blood cell: An important component of blood that helps the body protect against infection; also called leukocyte or leucocyte.
Assessment
Pre-Activity Assessment
Quiz: Have students begin class by completing a short bell-ringer quiz to identify the nature of mixtures (homogeneous or heterogeneous) from the separation technique used. The quiz is Task 1 in the Self-Guided Learning Module Handout.
Activity Embedded Formative Assessment
Student Handout: Embedded assessments are incorporated throughout the Handout, which guides students through the activity. Expect students to be consistently progressing as they move from one task to the next. Circulate through the group lab stations, monitoring and supporting students to aid their moving at a good pace. Refer to the Self-Guided Learning Module Handout Answer Key for a great amount of information, including answers to the Interim Summarization.
Day 1 Homework: The Task 8 homework assignment on the Handout has students think through their experimental designs in anticipation of Day 2 lab work. Review their answers to make sure they have attained the necessary understanding of centrifugation as acceleration sedimentation.
Review: On Day 2, before students get started with the lab, conduct a quick review to verify their understanding and address any misconceptions. Make sure every group is clear about the given materials and the labs they will be carrying out.
Post-Activity Assessment
Student Handout: Students complete the rest of the Handout as they work though the experimentation. Review their observations, inferences, answers and writing for Tasks 9-13 to gauge their comprehension of the activity concepts. Refer to the Answer Key.
Day 2 Homework: Tasks 12 and 13 on the Handout are homework assignments with two components: a literacy-related task and an industry-related task. Refer to the Answer Key for a great amount of information, including substantial concept information related to the research and writing assignments
Research-Based Activity (Literacy Skills): Building off the Task 12 assignment, verify student comprehension by leading a class discussion of the different types of centrifugation techniques including the principle behind each and the applications of each type.
College and Career Readiness Task: As assigned in Task 13, students evaluate the importance of the blood processing industry and analyze its career options. They list the pre-requisite educational qualifications, skills, attitude and personality required for jobs in this industry. If desired, lead a class discussion to further explore this material and check on student comprehension.
Analytical Creativity: Student assignment: If you were to model blood on your own, what materials would you use? Post your thoughts on the class blog, and respond to at least two other posts on this theme from your classmates. Your responses must reflect deep learning and commitment to seek, find and share. Review student posts to assess their depth of comprehension of the activity concepts.
Safety Issues
- While all materials used in this activity are benign, no material should be smelled, tasted or consumed.
- No material should be heated or burned.
- Require students to wear aprons and goggles when they conduct the lab work.
- Permit only the teacher to operate the centrifuge to minimize any risk to students.
Centrifuge Safety Tips for the Teacher
- Centrifuges are high-speed rotational instruments to separate out dispersed and suspended particles from solutions by spinning the solution in centrifuge tubes through the application of centrifugal force. Centrifuge tubes have a conical bottom that enables the collection of a small amount of the sample. Do not use cracked centrifuge tubes, which might break upon centrifugation.
- Keep the sample volume below half of the tube height or the liquid might overflow upon centrifugation. Wipe off any liquid on the outside of the tube, which might lead to corrosion of the machine's tube holder. For safety, insert pairs of centrifuge tubes in two holders located at opposite positions in the centrifuge; the weights of these sample tubes must be equal.
- Never open the cover or put a hand in the instrument during centrifugation. Remove all centrifuge tubes from the instrument when centrifugation is finished or the tubes not in use might cause an accident. If a centrifuge tube breaks in the tube holder, immediately turn off the machine, remove the broken glass and sample, then wash the tube holder with water and let it dry.
Troubleshooting Tips
- If the V8 beverage/olive oil/petroleum jelly mixture does not sediment at the expected rate, dilute the V8 to 80% by adding 20% water (for a 100 ml volume, combine 80 ml of V8 and add 20 ml of water) and then mix with the olive oil-petroleum jelly mix.
- When students are assembled in groups, a tendency exists for one or two students to not begin working, and instead wait for others team members to finish and then copy down other students' work. However, in this activity, every student must gain the depth of knowledge necessary to proceed to doing the lab, so it is important that the teacher ensure that each student individually completes all handout sections.
- To facilitate the cleaning process after the lab is completed, have available some long strips of filter paper (cut some paper towels into long thin pieces) for wiping away the remnants from the graduated test tube and the screw, centrifuge tubes and mixing vessel. Once oil has been removed almost completely with the filter paper, then washing becomes easy. Ask students to soak the test tubes in a bowl of soapy water. After a few minutes, scrub the test tubes with test tube brushes and rinse them under water. As long as the materials are not hazardous, get student help to tidy up the lab and put things back where they belong immediately after activity completion.
Activity Extensions
Modeling and executing ESR clinical tests to simulate various disease conditions: Direct students to conduct research about ESR tests and challenge them to create blood models that would have different ESR values, enabling correlation to different disease conditions. Ask them to delineate what alterations in the composition of the blood models bring about different sedimentation rates.
Activity Scaling
Adapt the activity as makes sense for student age and grade level, taking care that the learning objectives are met.
For higher grades:
- Have students explore new blood models using materials other than the V8 beverage. For example, one student (shown in Figure 1) prepared a blood model using beet juice and salt mixed with olive oil containing 1% mass/volume of petroleum jelly. She was able to recover petroleum jelly from olive oil only in centrifugation and not in sedimentation. Encouraging students to propose and test new models is a good exercise in divergent thinking.
- Ask students more higher-order post-lab inquiry questions, such as:
- What if mayonnaise was used instead of petroleum jelly? What might have happened to the mixture? Justify your proposition.
- What if you added about 5 ml of corn syrup into the V8 drink and conducted this experiment? What differences in observations would you expect? Justify your proposition.
- What if you added a pinch of corn starch to the V8 beverage? How would you expect the V8 to behave in the separation process?
- Ask students to explore whether studying the separation regimes of blood would shed light on the intricacies involved in understanding thrombotic events. To help students get an insight about thrombotic events, show two minutes of the Virchow's Triad video at https://www.youtube.com/watch?v=37uti7tzsYE. Then further reinforce that blood clots because certain blood components separate out of the blood owing to 1) the need to repair blood vessel injury, either a true injury or false injury due to the rupture of a fat deposit inside a blood vessel, 2) increased viscosity of the blood fluid, called hypercoagulability and 3) blood being agitated by turbulent forces.
For lower grades:
- Adapt the many tasks into individual activities; for example, have students focus on only completing Tasks 1 and 2, 1 through 3, or 1 through 4.
- Incorporate drawing and coloring more extensively. Have students draw and color the separation of blood in a test tube into two parts and in a centrifuge tube into three parts: use red for erythrocytes, yellow for plasma and gray for the buffy coat. Ask students to draw, color and label acceleration in a centrifuge due to centrifugal force. Or, ask students to diagrammatically represent how sedimentation transforms into centrifugation due to accelerating the gravitational force using the centrifuge.
- Employ alternate teaching methods to reinforce the concepts. For example, use total physical response (TPR) in an adapted musical chair exercise. Conduct a circling activity that is similar to the musical chair game in which students circle in three concentric circles. Students in the middle circle sit with their heads down while students in the outer and inner circles stand. When you whistle (or play some music), the outer and inner circle students start running. After a few seconds, the central circle students stand up in series and start to run along with the other circles. With suitable color distinction in caps or attire, this results in a very TPR activity for grades up to eight. Iconic representation: Ask students to use icons to represent the causes of thrombosis or clot formation. Plenty of free-to-use icons are available at: http://fortawesome.github.io/Font-Awesome/icons/.
- Ask students to write poems or raps about what they have learned, and present them to the class. If desired, record them and upload to YouTube to share.
Additional Multimedia Support
During Day 1, Task 3, students view the following YouTube videos and take notes:
- This Is What Snake Venom Does to Blood! (1:13 minutes) Shows the almost instantaneous coagulation (clotting) of blood by a drop of snake venom. https://www.youtube.com/watch?v=4WvnjCkLbvY
- Milk Curdling: A Fun, At-Home Science Experiment (1:28 minutes) Shows the coagulation (curdling) of milk by citric acid (lemon juice). https://www.youtube.com/watch?v=m9sIBgfllFs
- Blood Testing Facts: Blood Test Tube Separation (2:05 minutes; blood testing facts at 37 to 60 seconds) In a blood testing lab, shows how blood samples are contained in test tubes, with or without anti-coagulant, for the separation of plasma or serum. https://www.youtube.com/watch?v=wCyug61-r_c
- Video title: Sedimentation by Richard Holdich (4:37 minutes; view only 0 to 29 seconds for basic sedimentation) An animation with voice over that shows the sedimentation process; the effect of solute size, mass and concentration is explained. https://www.youtube.com/watch?v=E9rHSLUr3PU
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students demonstrate the erythrocyte sedimentation rate test (ESR test) using a blood model composed of tomato juice, petroleum jelly and olive oil. They simulate different disease conditions, including rheumatoid arthritis, anemia, leukocytosis and sickle-cell anemia, by making appropriate variatio...

Students learn how to classify materials as mixtures, elements or compounds and identify the properties of each type. The concept of separation of mixtures is also introduced since nearly every element or compound is found naturally in an impure state such as a mixture of two or more substances, and...

To gain an understanding of mixtures and the concept of separation of mixtures, students use strong magnets to find the element of iron in iron-fortified breakfast cereal flakes. Through this activity, they see how the iron component of this heterogeneous mixture (cereal) retains its properties and ...

Students gain a better understanding of the different types of materials as pure substances and mixtures and learn to distinguish between homogeneous and heterogeneous mixtures by discussing an assortment of example materials they use and encounter in their daily lives.
References
Dean, Lauren. Blood Groups and Red Cell Antigens. Bethesda, MD: National Center for Biotechnology Information, 2005. Accessed December 2014. http://www.ncbi.nlm.nih.gov/books/NBK2261/?depth=2
Lower, Stephen. "Matter under the Microscope." Last updated November 17, 2009. General Chemistry Virtual Textbook > States and Solids. Accessed December 2014. http://www.chem1.com/acad/webtext/states/states.html
Stec, Theresa C. "What is in the Bag?" Accessed December 2014. (34-slide PDF file; an overview of blood and blood products) http://c.ymcdn.com/sites/www.apheresis.org/resource/collection/387FC8D3-D586-4DC2-A60D-EA1A83285A68/Fri_1515._2_ES_V_Stec_Seacliff_A_&_B_update.pdf
"The Structure and Functions of Blood." 2003. IvyRose. Accessed December 2014. http://www.ivyroses.com/HumanBody/Blood/Blood_StructureandFunctions.php
Copyright
© 2015 by Regents of the University of Colorado; original © 2014 Georgia Institute of TechnologyContributors
Renuka RajasekaranSupporting Program
Partnerships for Research, Innovation and Multi-Scale Engineering (PRIME) RET, Georgia TechAcknowledgements
This work was carried out by the author as a part her PRIME GIFT summer internship at the Bioengineering Division of Georgia Institute of Technology under the guidance of David Ku.
This activity was developed by the Partnerships for Research, Innovation and Multi-Scale Engineering (PRIME) Research Experience for Teachers (RET) Program at Georgia Institute of Technology, funded by National Science Foundation RET grant no. EEC 140718. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: July 20, 2023






User Comments & Tips