Quick Look
Grade Level: 10 (9-12)
Time Required: 1 hours 30 minutes
Conduct over two days since students make soap in Part 1, which needs to sit for 24-48 hours before completing Parts 3 and 4. Shorten activity by purchasing liquid soap and shampoo.
Expendable Cost/Group: US $10.00 The activity also uses some non-expendable items, such as lab equipment; see the Materials List for details.
Group Size: 2
Activity Dependency: None
Subject Areas: Chemistry
NGSS Performance Expectations:

| HS-PS2-6 |
Summary
Students learn about the properties of solutions—such as ion interactions, surface tension and viscosity—as they make their own soap and shampoo and then compare their properties. Working as if they are chemical engineers, they explore and compare how the two surfactants behave in tap water, as well as classroom-prepared acidic water, hard water and seawater using four tests: a “shake test” (assessing the amount of bubbles produced), a surface tension test, a viscosity test, and a pH test. Then they coalesce their findings into a recommendation for how to engineer the best soap versus shampoo. The activity may be shortened by using purchased liquid soap and shampoo from which students proceed to conduct the four tests. A lab worksheet and post-quiz are provided.Engineering Connection
Chemical engineers in the soap industry must have a clear understanding of the topics explored in this activity—including pH, viscosity, surface tension and ion interactions—in order to design surfactants for specific applications, ranging from soaps and shampoos to detergents. In this activity, students engineer soaps and shampoos and test their surfactants in different types of water. Similarly, chemical engineers systematically test their designs before making the products ready for the consumer market.
Learning Objectives
After this activity, students should be able to:
- Describe surfactant behavior in tap water, hard water, salt water and acidic water.
- Compare and contrast soap and shampoo properties.
- Describe surface tension and viscosity.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS2-6. Communicate scientific and technical information about why the molecular-level structure is important in the functioning of designed materials. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Communicate scientific and technical information (e.g. about the process of development and the design and performance of a proposed process or system) in multiple formats (including orally, graphically, textually, and mathematically). Alignment agreement: | Attraction and repulsion between electric charges at the atomic scale explain the structure, properties, and transformations of matter, as well as the contact forces between material objects. Alignment agreement: | Investigating or designing new systems or structures requires a detailed examination of the properties of different materials, the structures of different components, and connections of components to reveal its function and/or solve a problem. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the role of troubleshooting, research and development, invention and innovation, and experimentation in problem solving.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Mississippi - Science
-
Analyze the relationship between microscopic and macroscopic models of matter.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
For the teacher’s introductory demonstration:
- dropper
- water and alcohol, several drops of each
Each group needs:
- three 250-ml beakers
- thermometer
- glass stirring rod
- hot plate
- pH meter; alternatively, use pH paper
- 50-ml graduated cylinder
- pipette
- 4 test tubes with caps
- penny
- 50 g olive oil
- 6.7 g potassium hydroxide (KOH)
- 50 ml of 5% triethanolamine
- 1 drop of phenolphthalein
- 5% oleic acid in ethanol solution, 5 to 10 drops
- 100 ml deionized (DI) water
- 1 ml tap water
- 1 ml each of acidic water, hard water and salt water; prepared as follows (plenty for the entire class):
- acidic water: 700 ml of distilled water + 300 ml of vinegar
- hard water: 1 liter of distilled water + 70 grams of CaCl2 (calcium chloride)
- salt water/seawater: 1 liter of distilled water + 62 grams of NaCl (salt)
- 12 x 12-inch (~30 x 30-cm) polypropylene sheet, such as cut from a 24 x 36-inch (61 x 91-cm) Twinwall Plastic Sheet for $8.61 at Home Depot
- a way to label beakers
- drain or bucket for viscosity test
- stopwatch, or other timer
- (optional) fragrance to add to homemade soap, such as essential oils
- Soap vs. Shampoo Lab Worksheet, one per student
- Soap vs. Shampoo Post-Lab Quiz, one per student
Note: Chemicals are available from chemical supply vendors like Flinn Scientific, as well as Amazon.com.
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/usm_surfactant_activity1] to print or download.Pre-Req Knowledge
A familiarity with polarity/electronegativity and how to make homogeneous solutions.
Introduction/Motivation
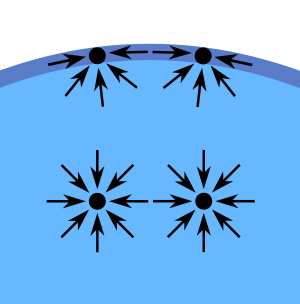
What is surface tension? (Listen to student definitions.) Surface tension is the tension on the surface of a liquid caused by the attraction of the molecules. Thanks to surface tension, some insects, such as water striders, as well as basilisk lizards, can walk or run on top of water without falling in!
(Demonstrate how the surface tension of alcohol and water compare by placing a drop of each side by side. Explain how the surface tension of each relates to the droplet shape. Draw attention to the dome shape the water drop makes.) Take a look at these droplets; one is alcohol and one is water. Why does a drop of water make a dome shape? (Listen to student ideas.) Water is a bent polar molecule so the water molecules are attracted to one another; since this attraction is strong, the shape bends.
(Point out the flat shape of the alcohol drop.) Why does the drop of alcohol make a flat shape? (Listen to student ideas.) The polarity and shape of alcohol molecules are different from those of water, and they break the surface tension.
What else can break the surface tension of water? (Expect students to suggest cleaning products, soaps, shampoos and other aqueous solution.) That’s right, the molecular shapes of cleaning products helps to break the surface tension of water.

How do soaps and shampoos “clean?” (Listen to student explanations.) Cleaning products form micelles, lipid molecules that take a spherical shape around molecules they are attracted to, such as dirt. These micelles also break the surface tension of water and form suds.
What makes soap and shampoo different from each another? What would happen if you washed your hair with soap instead of shampoo? (See if students have any ideas.) Soap has a higher pH than shampoo; it would dry out your hair. This example shows how it is important that chemical engineers working in the soap industry design shampoo and soap with the appropriate pH levels.
In this activity, you will work as chemical engineers as you learn how these cleaning products are made, how they differ, and how to apply terms such as viscosity, surfactant, surface tension and pH.
Procedure
Teacher Background
Surface tension exists because the molecules inside a liquid experience roughly equal cohesive forces in all directions, but molecules at the surface experience larger attractive forces toward the liquid than towards the gas (such as air). Surfactants are used to break the surface tension of water. Cleaning products, such as soap and shampoo, are examples of surfactants. They work by forming micelles that attract to the polar or nonpolar molecules in water. The ideal pH of shampoo is generally ~5.5, while the pH of liquid soap ranges from ~7-10. The higher the pH, the dryer the product makes a person’s hair. In this activity, students learn how these cleaning products are made, how they differ, and how to apply terms such as viscosity, surfactant, surface tension and pH.
Before the Activity
- Gather materials and make copies of the Soap vs. Shampoo Lab Worksheet and Soap vs. Shampoo Post-Lab Quiz, one each per student.
- Prepare the acidic water, hard water and salt water using the proportions provided in the Materials List.
- Alternative setup to simplify/shorten the activity: Purchase liquid soap and shampoo instead of having students make those surfactants. Then jump into the testing and analysis portions of the activity.
- (optional, but recommended) Work through the lab worksheet on your own, making the soap and shampoo, and then testing your surfactants. Your experiences in doing this will prove valuable in answering student questions and guiding them through the activity steps, as well as providing you with an answer key for expected test data and giving you an understanding of what students will find, based on the nature of your local tap water.
With the Students
- Facilitate the pre-assessment small-group discussions, as described in the Assessment section.
- Present to the class the Introduction/Motivation content, which includes showing students a drop of alcohol and a drop of water for comparison purposes.
- Hand out the worksheet.
- Divide the class into groups of two students each.
- Explain to the class: This activity is composed of four main parts. Each group will: 1) make liquid soap (or skip this step if using purchased liquid soap), 2) make shampoo (or skip this step if using purchased shampoo), 3) explore both surfactants using a series of four tests, and 4) analyze your results and answer questions based on your findings, including making a recommendation.
- Guide students through worksheet Part 1: Making Liquid Soap. Circulate the classroom and be available to answer questions. Students will:
A. Weigh out 50 g of olive oil and pour into a 250 ml beaker.
B. Place the beaker with oil onto a hot plate and keep the temperature between 33-43 °C. (Alert students to monitor the oil closely. When dealing with oils, a slow rise in temperature is better than a fast one. Advise students to be patient.)
C. Pour 17.5 ml of water into a different 250-ml beaker.
D. In the same beaker, slowly add 6.7 g of KOH while stirring with a stirring rod until completely dissolved. Explain to students that this is the “base solution.” Upon mixing the KOH in water, the solution might feel warmer. After it has dissolved, have students check the temperature. Expect the temperature to also be between 33-43 °C. If not, direct students to place the beaker on the hot plate as well and monitor it.
E. Once both solutions are at the same temperature, slowly pour and stir the base solution into the olive oil. Continue to stir for 10 minutes or until the solution has traced (a term for the thickening of the soap/lye solution). Expect the mixture to stick slightly to the stirring rod when lifted. (Optional; once the soap mixture has reached the tracing stage students may add fragrance or additives.)
F. Let the soap sit for 24-48 hours and make sure not to use the soap until the pH has been tested!
- Next, guide students through worksheet Part 2: Making Shampoo. As before, circulate the classroom and be available to answer questions. Students will:
A. Pour 50 ml of 5% triethanolamine in water into a 250-ml beaker.
B. Add 1 drop of phenolphthalein to the solution and gently stir. The solution should have a pink color.
C. Add dropwise the 5% oleic acid in ethanol solution until the solution becomes colorless (5 to 10 drops). Be sure you are stirring while you add the solution.
- When the soap and shampoo are ready, guide students to complete worksheet Part 3: Testing Surfactants. Following the worksheet instructions, students complete the following four tests:
- Shake Test: The higher the height of the bubbles in the test tube, the better the cleaning in that type of solution (see Figure 1).
- Penny Test: The more drops of solution the penny can hold, the higher the surface tension.
- Viscosity Test: The faster the flow of droplets, the less tension each solution has. Expect students to note a correlation between the viscosity and penny test results.
- pH Test: Shampoo has a lower pH than soap. (The ranges vary, depending on the type and whether they are pH-balanced or not, which the teacher discovers by testing each before the lab. Also deionized water may read below pH 7.)

- Give students time to complete worksheet Part 4: Results Analysis and Recommendation.
- Engage the class in a post-lab discussion. As a class, review the data students gathered and ask the following questions:
- What ions are in hard water that cause dry skin? (Answer: Calcium chloride.)
- How can we tell if water has a high surface tension? (Answer: The higher the surface tension, the more drops that can be held together when on the surface of a penny.)
- What can break surface tension? (Answer: Impure solutions, bigger molecules, different polarities.)
- How is viscosity measured? (Answer: Viscosity is the resistance of flow of a liquid. The higher the viscosity, the slower it flows. Viscosity is measured by the amount of volume per unit of time.)
- What test shows the difference in shampoo and soap? (Answer: A pH test.)
- How can soaps and shampoos affect the water quality of the environment? (Answer: Even though most surfactants are toxic, soaps and shampoos have little effect due to their structure and buffers included in them.)
- Why is it important to engineer soap and shampoo correctly? (Answer: A wrong combination could cause environmental problems or prove toxic to people and other organisms.)
- Administer the post-activity quiz.
Vocabulary/Definitions
micelle: A lipid molecule that take a spherical shape around molecules it is attracted to, such as dirt.
pH: A measurement of the acidity or alkalinity of a solution.
surface tension: The elastic tendency of the exposed surface of a liquid that makes it acquire the least surface area possible.
surfactant: A substance that tends to reduce the surface tension of a liquid in which it is dissolved.
viscosity: A measure of a fluid’s resistance to flow.
Assessment
Pre-Activity Assessment
Discussion Questions: In small groups, have students discuss the following:
- Have you ever been to a place where the water felt, smelled or tasted different than you were used to? Where were these locations? (Possible answers: Theme park, camp, beach, a different house, city or country.)
- What compounds do you expect to be in your water supply? (Possible answers: Sodium chloride, calcium chloride, fluoride, various minerals.)
- Why do you think water filtration is important for communities? How could not having clean water affect a community? (Possible answers: Sanitation, human health, disease prevention, water-borne sickness.)
Activity Embedded Assessment
Lab: Have groups work through the activity, guided by the instructions on the Soap vs. Shampoo Lab Worksheet, answering questions and filling in the data table. Circulate the classroom and monitor student progress to ascertain students’ depth of understanding. Review students’ final worksheet answers, too.
Post-Activity Assessment
Post-Lab Discussion: After the lab is completed and worksheets are finished, engage the class in a discussion to share and review results and conclusions. Ask the questions provided in the Procedure section.
Post-Lab Quiz: To conclude, administer the five-question Soap vs. Shampoo Post-Lab Quiz. Review student answers to assess their individual takeaways.
Safety Issues
When making soap or shampoo, be cautious in permitting students to handle the KOH and triethanolamine. Read the chemical labels carefully before using.
Troubleshooting Tips
Check the pH of any shampoo and liquid soap before doing the lab with students. The pH can vary, depending on the type of shampoo or liquid soap.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about the basics of molecules and how they interact with each other. They learn about the idea of polar and non-polar molecules and how they act with other fluids and surfaces. Students acquire a conceptual understanding of surfactant molecules and how they work on a molecular level. ...

Student teams are challenged to evaluate the design of several liquid soaps to answer the question, “Which soap is the best?” Through two simple teacher class demonstrations and the activity investigation, students learn about surface tension and how it is measured, the properties of surfactants (so...

Students culture cells in order to find out which type of surfactant (in this case, soap) is best at removing bacteria. Groups culture cells from unwashed hands and add regular bar soap, regular liquid soap, anti-bacterial soap, dishwasher soap, and hand sanitizer to the cultures.

Students are presented with the question: "Why does a liquid jet break up into droplets?" and introduced to its importance in inkjet printers. A discussion of cohesive forces and surface tension is included, as well as surface acting agents (surfactants) and their ability to weaken the surface tensi...
References
Amato, Dahlia N.; Douglas V. Amato, Jananee Narayanan, Brian Donovan, Jessica R. Douglas, Susan E. Walley, Alex S. Flynt and Derek L. Patton. (2015) “Functional, Composite Polythioether Nanoparticles via Thiol-Alkyne Photopolymerization in Miniemulsion.” Chemical Communications, Royal Society of Chemistry. Issue 51, June 2015, pp. 10910-10913. (Source of background research of polymerizable surfactants, with some adaptation.) http://pubs.rsc.org/en/Content/ArticleLanding/2015/CC/c5cc03319e#!divCitation
Dux, Emma. (2013) “Two in One: The Chemistry of Shampoo and Conditioner.” Chemistry Review. February 2013, pp. 6-10. (Source of the why shampoo is different from soap.) https://www.hoddereducation.co.uk/media/Documents/Magazines/Sample%20Articles/CR-V22.pdf
Goniometer. Last updated June 22, 2016) In Wikipedia, The Free Encyclopedia. Accessed July 7, 2016. (Source of explanation of surface tension.) https://en.wikipedia.org/w/index.php?title=Goniometer&oldid=726480494
Patterson, Jim. World of Chemistry. North Seattle Community College, WA. Accessed June 20, 2015. (Source of some lab background on soap synthesis, with some adaptation.) http://facweb.northseattle.edu/jpatterson/
West Virginia State University. Accessed June 20, 2015. (Source of some lab background on the shake test and shampoo synthesis, with some adaptation.) https://online.wvstateu.edu/access/content/group/78db4edb-6dd8-419a-bb16-ce8369de5034/Uploaded%20Fall%20files/201202_CHEM_100_06_1900/C100%20lab%204.pdf
Copyright
© 2016 by Regents of the University of Colorado; original © 2015 University of Southern MississippiContributors
Mark Holcomb; Dahlia Amato; Douglas Amato; Derek L. PattonSupporting Program
Research Experience for Teachers Program, School of Polymers and High Performance Materials, University of Southern MississippiAcknowledgements
This activity was developed under the Research Experiences for Teachers (RET) in Engineering and Computer Science Site for Sustainable Polymer Engineering Research program in the University of Southern Mississippi’s School of Polymers and High Performance Materials, funded by National Science Foundation RET grant no. EEC 1406753. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: May 12, 2021





User Comments & Tips