Summary
Through a teacher-led class demo, students observe a simple water cycle model to better understand its role in pollutant transport. Using kitchen or lab equipment, the demo simulates a point source of pollution in a lake and the resulting environmental consequences—one way in which pollution is affected by the water cycle. A student worksheet is provided.Engineering Connection
Once a pollutant is introduced into the environment, it is possible, but generally very difficult, to clean up. In some situations, bioremediation, or cleaning up pollutants through the use of microbes (bacteria, plants), is the best approach. Civil and environmental engineers design the distribution systems and bioreactors to implement the bioremediation process using specific microbes identified by biochemists to remove or neutralize contaminants in soil or water. Similarly, civil engineers design water and waste treatment plants to assure clean water in every community.
Learning Objectives
After this activity, students should be able to:
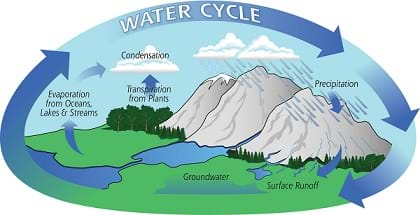
- Define and provide examples of evaporation, condensation and precipitation.
- Understand and explain how a simple water cycle model can be used to model pollution transport.
- Understand and explain how the water cycle is related to air pollution.
- Begin to understand some ways that engineers use and interact with the water cycle.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
5-ESS2-1. Develop a model using an example to describe ways the geosphere, biosphere, hydrosphere, and/or atmosphere interact. (Grade 5) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model using an example to describe a scientific principle. Alignment agreement: | Earth's major systems are the geosphere (solid and molten rock, soil, and sediments), the hydrosphere (water and ice), the atmosphere (air), and the biosphere (living things, including humans). These systems interact in multiple ways to affect Earth's surface materials and processes. The ocean supports a variety of ecosystems and organisms, shapes landforms, and influences climate. Winds and clouds in the atmosphere interact with the landforms to determine patterns of weather. Alignment agreement: | A system can be described in terms of its components and their interactions. Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-ESS2-4. Develop a model to describe the cycling of water through Earth's systems driven by energy from the sun and the force of gravity. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to describe unobservable mechanisms. Alignment agreement: | Water continually cycles among land, ocean, and atmosphere via transpiration, evaporation, condensation and crystallization, and precipitation, as well as downhill flows on land. Alignment agreement: Global movements of water and its changes in form are propelled by sunlight and gravity.Alignment agreement: | Within a natural or designed system, the transfer of energy drives the motion and/or cycling of matter. Alignment agreement: |
State Standards
Colorado - Science
-
Use evidence to model how water is transferred throughout the earth
(Grade
6)
More Details
Do you agree with this alignment?
Materials List
To create one ecosystem:
- 12-inch frying pan
- glass bowl; one with a narrow base works best
- aluminum pie tin
- 1 quart of cubed or crushed ice (not block ice)
- water
- small household fan, 4-6-inch diameter is best
- hot plate or stove top
- food coloring; dark color is best
- (optional) aluminum foil
- Water Cycle Worksheet, one per student
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_air_lesson05_activity1] to print or download.Introduction/Motivation
Once a pollutant is introduced into the environment, it is possible, but generally very difficult, to clean up. Engineers are very helpful in this regard. Bioremediation, or cleaning up pollutants through the use of microbes, is a growing field. While biochemists are responsible for determining the microbe to use for a specific task, civil or environmental engineers design the distribution systems and bioreactors to implement the bioremediation.
The ground water in Tuba City, AZ, became contaminated from the tailings of a nearby uranium mine. Engineers at States Filter Corporation designed an evaporation system to cleanse nearly one billion gallons of groundwater. Their evaporation system does, in effect, just what this activity does: It heats polluted water so that the water evaporates away and leaves the pollutants behind. The system designed for this cleanup process is vastly more efficient than the simple process used in this class demonstration. In Tuba City, water is evaporated in a vacuum, which eliminates atmospheric pressure and thus lowers the boiling point of the water to approximately 140 ºF. Next, the evaporated water is condensed in such a way that the heat from the gaseous water is removed and returned to help heat liquid water, conserving even more energy. The clean water is returned to the aquifer, while the concentrated waste-water is directed to large evaporation ponds, where the water is evaporated by the Arizona sun, and the pollutants left behind—exactly as in this activity! This is just one example of the many ways that engineers work to repair the damage caused by pollution.
Procedure
Before the Activity
- Find an area or workspace in which all students have a clear view since this activity is a demonstration to show the entire class the water cycle.
- Gather materials and make copies of the Water Cycle Worksheet, one per student.
With the Students
- Place the frying pan on the hotplate filled with water, but do not turn on the hotplate. Explain to the students that this is the "lake" for your ecosystem.
- Fill the glass bowl with ice and water. Explain to the class that you are going to hold the bowl of ice water above the boiling water. Explain to students that this will be the "cloud" for your ecosystem.
- (Optional) Use the aluminum foil to create a "watershed" underneath the "cloud." Set up a channel of foil so that any water falling from the cloud drips onto the watershed and can be directed into the aluminum pie tin ("reservoir").
- Hold the bowl ("cloud") above the pan, but slightly off-center. Position the aluminum pie tin beneath the cloud (or "watershed" if you made one) to act as a reservoir.
- Set up the fan approximately two feet from the frying pan so as much steam as possible hits the "cloud" (optimize this distance once steam is being formed). This serves as the "wind."
- Turn on the hot plate and watch for steam. While the water heats, it is a good time to ask students what they know about the water cycle and what they think will happen in the experiment. Explain that the hot plate heats the water so that it evaporates into the air — just like the sun dries up a puddle. The steam condenses on the bottom of the bowl of ice. This is just how a cloud is formed when water droplets condense in the air. The winds blow the small drops around so that they collide with one another. During these collisions, some drops combine with others making bigger and bigger drops. When the drops become so large that the winds cannot keep them in the sky, the drops fall as rain or snow, known as precipitation. This is similar to the large drops falling from the bottom of the bowl.
- Once steam starts to form, turn on the fan ("wind"), and adjust its position so that it gently blows the steam towards the bowl ("cloud"). Once the water is boiling, hold the bowl of ice over the steam. The steam condenses on the bottom of the bowl. (You want the water to drip into the foil when it condenses.)
- If all goes well, the steam is blown by the fan to the cloud, condenses on the cloud, drips to the watershed, and flows into the reservoir. It may take a while to get a significant amount of water in the reservoir.
- Take some time to observe and share what is happening (see Figure 1). Ask some questions: What is happening to the boiling water? What do you see happening on the bottom of the bowl? What do you see happening in the bowl of ice water? How does the water get on the outside of the bowl? Are the water drops on the side of the bowl the same size? Why? Which drops are falling from the bowl? Why? Which drops look like rain? Which drops look like a cloud? How are the big drops formed?

- Put a few drops of food coloring, representing pollution, into the frying pan, representing the lake, and one drop in the aluminum pie tin, representing a reservoir. Describe this as a simulation of some type of point source of pollution, such as a factory, manufacturing plant or illegal dumping (see Figure 2).

- Let the cycle continue until a significant amount of water accumulates in the reservoir. Have students watch the color change in the reservoir as it progresses.
- Ask if the water collected in the reservoir is colored anymore. (It should not be!) Ask why this is a GOOD thing. (Answer: The water cycle can help dilute some pollution in certain areas. It also means that the pollution from the lake was not transported to the reservoir—great news!).
- Ask why this is also a BAD thing. If students get stuck, ask them to look in the frying pan after most of the water has boiled out (noticing that a lot of food coloring is left behind). (Answer: Many pollutants DO NOT evaporate with water and follow the water cycle—this is bad because the pollutants get concentrated in whatever lake/pond/bay/river they are dumped in. After they have built up, they can be VERY harmful to the ecosystem.)
- Ask the students to imagine what would happen if you blew some dust into the air around the "rain" falling from the bowl (or other air pollution). Where would the dust go? What would its path be? Explain that many pollutants are transported via the methods in this demonstration: some are released into the air by evaporating water, some are carried by wind, some condense with water vapor and fall as acid rain.
- Explain that environmental engineers are concerned about the water cycle because pollutants can be carried along with the water—moving the pollutants from the air to the ground, into bodies of water and possibly back into the air with evaporation.
- Ask the students: What does air pollution have to do with the water cycle? Ask the students to complete the Water Cycle Worksheet, identifying the stages of the water cycle process, starting with evaporation. Assign the worksheet as homework and/or review as a class.
Vocabulary/Definitions
aquifer: An underground bed or layer of earth, gravel or porous stone that yields water.
bioreactors: An apparatus for growing organisms that are used for various purposes, including the bioconversion of organic waste.
bioremediation: The use of biological agents, such as bacteria or plants, to remove or neutralize contaminants, as in polluted soil or water.
condense: To change from a gas or vapor into a liquid. The opposite of evaporation.
evaporate: To change from a liquid into a gas or vapor. The opposite of condensation.
microbes: A tiny life form, a microorganism.
precipitation: Any form of water, such as rain, snow, sleet or hail that falls to the Earth's surface.
reservoir: A natural or artificial pond or lake used for the storage and regulation of water.
uranium: A heavy, silvery-white metallic element, radioactive and toxic.
watershed: A ridge of high land dividing two areas that are drained by different river systems; water parting; the region draining into a river, river system or other body of water.
Assessment
Pre-Activity Assessment
Prediction: Ask the students what they know about the water cycle and what they think will happen in the class demonstration. Solicit, integrate and summarize student responses.
Activity Embedded Assessment
Question/Answer: During the activity, ask the students the following questions to assess their understanding.
- What is happening to the boiling water? What do you see happening on the bottom of the bowl? What do you see happening in the bowl of ice water? How does the water get on the outside of the bowl? Are the water drops on the side of the bowl the same size? Why? Which drops are falling from the bowl? Why? Which drops look like rain? Which drops look like a cloud? How are the big drops formed?
- What would happen if you blew some dust into the air around the "rain" falling from the bowl (or other air pollution)? Where would the dust go? What would its path be?
Worksheet: Ask students to complete the Water Cycle Worksheet, assigning it as homework and/or review together in class.
Post-Activity Assessment
Diagramming: Ask the students to illustrate the water cycle concept through drawing. After watching the class demonstration, have them make a drawing of the water cycle that includes the lake, cloud, watershed and reservoir. They can use blue for the water and precipitation, and include arrows to show the path of the pollution transport. For a more detailed diagram, have the students label when in the cycle evaporation, condensation and precipitation take place.
Safety Issues
Remember to use caution when using the hot plate (or stovetop) with students.
Troubleshooting Tips
Preparation time is about 10 minutes. It takes about 20-30 minutes to get significant condensation ("rain") depending on the environmental conditions.
Allow time for the water to come to a boil. It will take a couple minutes for the water to condense on the bowl of ice water and start dripping.
Activity Extensions
Bring in a frozen bottle (clear) of a favorite student drink (such as red Kool-Aid). On a humid day, clear water condenses on the outside of the bottle while it is sitting on the desk. Explain that the red Kool-Aid is not leaking from the bottle, but that what is condensing (changing from water vapor to liquid) around the bottle is water present in the air.
Investigate different types of pollutants that are easily/not so easily transported via the water cycle.
Try the demonstration with an invisible pollutant. Make a salt-water solution and place that in the frying pan "lake." What happens when most of the water has evaporated from the pan? Does the water in the reservoir taste salty?
Have students research to find out how polluted certain lakes are, or what concentrations of pollutants in a lake are considered acceptable. For example, fewer than 25ppb (parts per billion) of one pollutant may be considered acceptable, while more than 100ppb might be considered hazardous.
Activity Scaling
- While this demonstration is appropriate for all age levels, modify the discussion to fit the needs of your students.
- For younger students, if they need help with the Water Cycle Worksheet, have them identify five stages: Evaporation from the ground, wind transport, condensation into clouds, precipitation and water returned back to Earth.
- While this activity is intended as a class demonstration, closely supervised older students may be able to do this on their own.
- Instead of using the Water Cycle Worksheet, have more advanced students draw their own diagram of the water cycle from watching the class demonstration.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn the fundamentals of using microbes to treat wastewater. They discover how wastewater is generated and its primary constituents. Microbial metabolism, enzymes and bioreactors are explored to fully understand the primary processes occurring within organisms.

Students learn about a special branch of engineering called bioremediation, which is the use of living organisms to aid in the clean-up of pollutant spills. Students learn all about bioremediation and see examples of its importance. In the associated activity, students conduct an experiment and see ...

Students examine in detail the water cycle components and phase transitions, and then learn how water moves through the human-made urban environment. Students show their understanding of the process by writing a description of the path of a water droplet through the urban water cycle, from the dropl...
References
Valenti, Michael. Cleaning Up After Industry. Updated 1999. American Society of Mechanical Engineers. Accessed July 22, 2004. Originally found at: http://memagazine.asme.org/
Copyright
© 2004 by Regents of the University of ColoradoContributors
Amy Kolenbrander; Alejandro Reiman-Moreno; Janet Yowell; Natalie Mach; Tyman Stephens; Malinda Schaefer Zarske; Denise W. CarlsonSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under grants from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation (GK-12 grant no. 0338326). However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: October 25, 2020






User Comments & Tips