Quick Look
Grade Level: 5 (3-5)
Time Required: 1 hour
Lesson Dependency: None
Summary
Through the use of models and scientific investigation, students explore the causes of water pollution and its effects on the environment. Through the two associated activities, they investigate filtration and aeration processes that are used for removing pollutants from water. They also learn about the role of engineers in water treatment systems.Engineering Connection
To benefit society, engineers design and build systems to keep our global water supplies clean. This requires that engineers investigate the water source, and route it travels before it is used by people, and study previous methods that have successfully cleaned our water supplies.
Learning Objectives
After this lesson, students should be able to:
- Explain the role of engineers in water treatment systems.
- Describe how contaminants leach into the soil and ground water and how they are absorbed by plants.
- Explain the natural water filtration process.
- Examine how much water is consumed by humans in a lifetime.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
Common Core State Standards - Math
-
Fluently multiply multi-digit whole numbers using the standard algorithm.
(Grade
5)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Explain why responsible use of technology requires sustainable management of resources.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
State Standards
Colorado - Math
-
Add, subtract, multiply, and divide decimals to hundredths.
(Grade
5)
More Details
Do you agree with this alignment?
Colorado - Science
-
Develop and communicate a scientific explanation addressing a question of local relevance about resources generated by the sun or Earth
(Grade
5)
More Details
Do you agree with this alignment?
-
Analyze and interpret a variety of data to understand the origin, utilization, and concerns associated with natural resources
(Grade
5)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/cub_environ_lesson06] to print or download.Introduction/Motivation
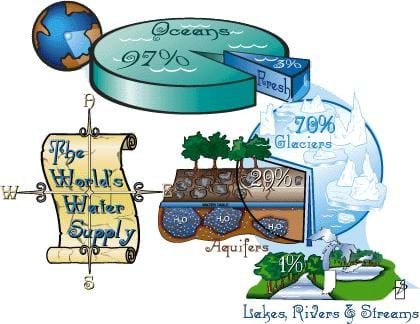
Did you know that 97% of the water on Earth is salt water? The other 3% is fresh water, but of this amount, 2% is frozen in ice caps. So, that means that only 1% of the Earth's water is available for drinking and growing food.
On average, Americans use approximately 88 gallons of water per person per day. The U.S. population keeps growing, and so does the amount of water use. The majority of water used by Americans goes through a waste water treatment facility designed by engineers and eventually returns to the water supply. This means — for the bulk of American residents — that the water you used this morning to brush your teeth travels through the water system, gets cleaned up at a treatment facility and returns to the community water supply and, subsequently, your own sink!
Water is essential to all life on Earth. The human body is composed of 60-70% water. Although much of the water in the body is recirculated, humans must have fresh water daily (obtained from eating and drinking) because some of a body's water is removed each day (through the natural processes of urination, breathing, perspiration, etc.).
Where does a body get its water supply? Name for me some liquids that people drink that contain water. (Write student suggestions on the classroom board.) List for me some foods that people eat that contain water. (Record these suggestions on the board.)
Water must be clean for humans and all other animals. Water pollution can cause poisoning and/or diseases if contaminated water or plants that grew from contaminated water are consumed. What are some sources of water pollution? (Write student ideas on the classroom board.)
Globally, water pollution is a big problem. The United Nations reports that at least 1.1 billion people do not have an adequate supply of drinking water. At least 30,000 people die everyday in the poorest parts of the world because of either a lack of water or unsafe drinking water (about 10 million each year).
Engineers explore and design ways to keep water clean. They study water supplies (where water comes from) and methods for cleaning water. Water engineers' goal, of course, is to develop processes that keep the water in our faucets — and everywhere else water is used — clean and healthy for the people who use it. What are some additional reasons why clean water is important? (Write student ideas on the board.) Following the lesson, students can put their design and problem solving skills to the test with the associated activity The Dirty Water Project: Design-Build-Test Your Own Water Filters.
Lesson Background and Concepts for Teachers
Interesting Water Facts
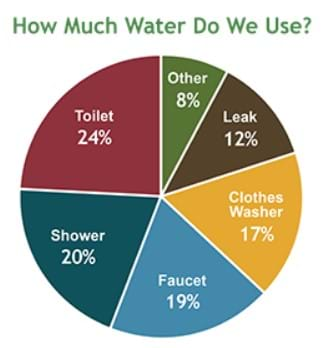
- The average American uses more than 300 gallons of water per day.
- A dripping faucet that leaks just one drop of water every second wastes 5 gallons of water a day (2,082 gallons in a year). Yet, a drip usually only costs a few dollars to repair.
- Irrigation has played a roll in human lives for nearly 5,000 years. Ancient Egyptian pharaohs ordered people to build channels to carry water to dry fields. In ~1,000 BC, a farmer with a bucket and a pole could irrigate about one-quarter acre a day. Then, in ~1,000 AD, a farmer using a buffalo to turn a wheel could irrigate about 5 acres a day. Today, in industrialized countries using advanced technology, a farmer can pump over 1,000 gallons of water per minute over a 160-acre area. On average, crops require about 2,200 lbs of water to produce 2.2 lbs of food.
- The Exxon Valdez, carrying millions of gallons of crude oil, ran aground in Prince William Sound, AK, in March 1989. More than 11 million gallons of oil spilled into the sea. It eventually spread across 1,100 miles of coastline and killed as many as 100,000 birds and 1,000 sea otters as well as other shore life.
- During the Gulf War in 1991, Iraq deliberately dumped 240 million gallons of crude oil from Kuwaiti oil reserves in an attempt to halt U.S. marine operations. Reports estimate that 8 million gallons ran directly into the Persian Gulf.
- Safe and abundant water is listed as fourth of the 20 greatest engineering achievements of the 20th century. (Source: National Academy of Engineers)
- Typhoid fever, cholera, dysentery and diarrhea killed many people in the early 1900s. Because of water treatment systems created by engineers starting in the 1940s in the U.S., these diseases were almost eradicated. Furthermore, distribution systems moved water into areas that were previously inhabitable. Students can learn more about compromised clean water with the associated activity What's Gotten Into You?
Causes of Water Pollution
Point source pollution is the leading cause of water pollution. Point source pollution is pollution from a definite source, such as: direct industrial discharge and dumping, accidents, deep-well injection, leaky landfills, leaking underground storage tanks, abandoned hazardous waste sites, septic tanks, etc. Point source pollution can usually be measured and strict government regulations control on its discharge (that is, permits must be obtained from the local government before discharging such pollutants into the environment). Following are some examples of how point source pollution exists today.
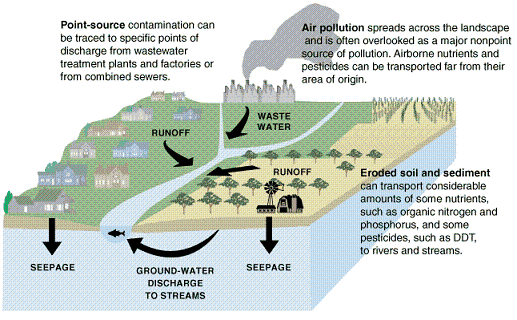
- City and Industrial Waste – Such waste includes chemical waste produced by factories, raw/partly treated sewage from cities (that contains dangerous, sometimes deadly, bacteria), and garbage that is irresponsibly dumped directly into bodies of water. This type of water pollution is the most heavily regulated today.
- Deep Well Injection – Industrial liquid waste is injected into porous areas that are sealed off from the aquifers above and below by a layer of impermeable rock. The rock can develop cracks, and sometimes the chemical wastes corrode through the impermeable rock. Also, the lining of the well can leak.
- Landfills – Leachate is a liquid produced when rainwater mixes with the substances that are buried in landfills. This liquid leaks from the landfill and often seeps into the groundwater.
- Heat – Some industrial companies use water to cool their equipment (like nuclear power plants). The water is heated above its normal temperature and is drained back into the original water source. This results in animals dying (when the temperature is outside their tolerance range) and an increase in algae growth — eutrophication — of the types that grow more quickly in warmer water.
- Saltwater Infiltration – When too much water is taken from freshwater aquifers near the ocean, salt water infiltrates the aquifers and contaminates them.
- Natural – This type of water pollution occurs in many ways including: blue-green algae that can poison fresh-water ponds, rapid algae growth called "red tides," dust/ash/heat from erupting volcanoes, germs (bacteria), and floods.
Nonpoint source pollution follows point source pollution in the amount of water pollution, but is still a major contributing factor. Nonpoint source pollution is described as water that runs over the ground and picks up — and then carries away — natural and human-made pollutants. Then this water is deposited into lakes, streams, coastal waters and underground sources of drinking water, resulting in harmful effects on drinking water supplies, recreation and wildlife. Nonpoint source pollution sources include agricultural runoff, urban runoff, mining, logging and grazing.

- Farmland Runoff – Irrigation runoff carries nitrates/phosphates (from fertilizer) and pesticide/insecticides to water sources. Runoff water also carries large quantities of soil that settle on the bottom of lakes and rivers.
- Transportation – This includes the use of waterways for industrial shipping (such as the Mississippi River) and the transportation of materials through pipelines (natural gases, liquids, oil or coal in a water "slurry" carried from Black Mesa Coal Mine, AZ, to Mojave, NV).
- Crude Oil - More than 50% of crude oil pollution comes from land-based, nonpoint sources — 40% from car owners changing their oil and improperly disposing of it; the rest from leaking oil pipes at oil production and transportation sites. About 10% of crude oil pollution comes from oil spills. The rest results from marine operations such as when ships clean their fuel tanks.
- Household Hazardous Waste Products – Annually, the average US household produces 530,000 tons of household hazardous waste products (chemicals, detergents, bleach, etc.) If improperly disposed, these can pollute the water system. Though safe disposal options of household hazardous waste has improved, in the early 1990's an estimated 300,000 tons of household hazardous waste products entered the water system each year by way of household drains or go unchecked into landfills. (Sources: 2012 Household Hazardous Waste, PSI; 1992 Environmental Almanac, World Resource Institute)
Effects of Water Pollution

Water pollution can cause diseases and poisoning in animals if they drink the contaminated water or eat the plants that grow from the contaminated water. In essence, pollution can halt their survival and reproduction.
Phosphates and nitrates in lakes, coastal waters, rivers, and wetlands throw off the natural balance so that too much food exists for plants and too little oxygen exists for animals. Such pollutants cause algae to grow too quickly, which uses up all the nutrients in the water. The algae then die and decompose, which uses up all the oxygen in the water. Subsequently, without oxygen the fish die. In large doses, nitrates may cause cancer in humans, which may result in death.
Oil spills may kill aquatic animals, and ultimately, the oil covers ocean floors and beaches. Such large amounts of oil interrupts organisms' ability to reproduce and prevents normal animal activities such as, oil-slicked feathers preventing birds from flying and staying warm.
Another effect of water pollution is in bodies of water used for recreation and commercial fishing. Severe pollution restricts commercial fishing because of the existence of contaminated fish and/or lack of aquatic life. And, recreational activities that involve water may not be enjoyed if pollution is present.
See an excellent history and timeline about water by the National Academy of Engineers at https://pressbooks.bccampus.ca/engineeringinsociety/chapter/greatest-engineering-achievements-of-the-20th-century-by-the-national-academy-of-engineering/
Associated Activities
- What's Gotten Into You? - Students use models to investigate the process and consequences of water contamination on land, groundwater and plants. This is a good introduction to building water filters found in the other associated activity, The Dirty Water Project: Design-Build-Test Your Own Water Filters.
- The Dirty Water Project: Design-Build-Test Your Own Water Filters - Students investigate different methods (aeration and filtration) for removing pollutants from water. They design their own water filters.
Lesson Closure
See http://sandiego.surfrider.org/ for tips on how minor everyday changes can help to prevent pollution. Emphasize that ordinary people can do ordinary things to prevent water pollution. Ask students to describe one thing they could do to help clean up water pollution.
Read the following poem "Recycled," by Verne N. Rockcastle. Then, discuss with students how the water they drank this morning may return to their children's glasses many years from now because of the planet's water cycle. Why might engineers need to know about this process?
Recycled
The glass of water you're about to drink
Deserves a second thought, I think.
For Avogadro, oceans and those you follow
Are all involved in every swallow.
The molecules of water in a single glass
In number, at least five times, outclass
The glasses of water in stream and sea,
Or wherever else that water can be.
The water in you is between and betwixt,
And having traversed is thoroughly mixed,
So someone quenching a future thirst
Could easily drink what you drank first!
The water you are about to taste
No doubt represents a bit of waste
From prehistoric beast and bird—
A notion you may find absurd.
The fountain spraying in the park
Could well spout bits of Joan of Arc,
Or Adam, Eve, and all their kin;
You'd be surprised where your drink has been!
Just think! The water you cannot retain
Will some day hence return as rain,
Or be held as the purest dew.
Though long ago it passed through you!
(Source: Wisconsin Department of Natural Resources http://dnr.wi.gov/org/caer/ce/eek/earth/groundwater/poem.htm)
Vocabulary/Definitions
aeration: The process of adding air to water, which is often done as part of the water purification process.
algal bloom: A period of excessive, unnatural growth of algae caused by an increase in nutrients (like phosphorus and nitrogen) in the water. It can lead to a decrease in the amount of oxygen available for other aquatic life.
aquifer: The space below the water table in which layers of soil and rock are saturated with water. Water in aquifers can generally be pumped out.
biochemical oxygen demand: The amount of oxygen required by an organism to perform biochemical functions. Abbreviated as BOD.
contaminants: Usually measured in parts per million (ppm) or parts per billion (ppb). This means one molecule of contaminant for every million (or billion) molecules of water. (For example, if you had a wealthy relative who dies and has $10,000,000 dollars to distribute. If you receive an inheritance of 5 ppb, then you get 5 cents!)
desalinization: A process that separates salt and water, leaving the saltwater drinkable.
eutrophication: A process by which the oxygen in a body of water is used up by the decaying bodies of microorganisms (algae with high BOD) that were stimulated into overgrowth by an abundance of nutrients. It is a common result of nitrate pollution.
groundwater: Water found between underground particles of soil and rock (how much depends on the permeability and porosity of the particles) that supplies wells and springs. It makes up approximately 97% of the freshwater on Earth. Some of the groundwater that we use today fell as rain hundreds or even thousands of years ago. Water falls to the earth and seeps through the particles of the soil that make up the crust of the Earth. It filters down until it reaches a water-saturated zone. The top of this zone is the water table and the part below it is called an aquifer.
leachate: Contaminated liquid produced by water seeping through solid waste (for example, rainfall seeping through a landfill).
nonpoint pollution: Pollution that cannot be traced to a single source because it comes from many different sources (for example, pesticides that wash off farm crops). This type of pollution is difficult to control and requires many people working together to make a change.
runoff: Water that "runs off" the surface of soil (into a sewer or body of water) instead of soaking into the ground.
water table: The top portion of an underground area where the soil and rock particles are saturated with water. The bottom portion is called an aquifer.
Assessment
Pre-Lesson Assessment
Brainstorming: Have students generate a number of possible ideas about the following topics. Encourage wild ideas and discourage criticism of any ideas.
- Name the various liquids that people drink that contain water. Record student suggestions on the board. (Example answers: milk, juice, coffee, tea, root beer, etc.)
- List different foods that people eat that contain water. Record student answers on the board. (Example answers: fruits, vegetables, meat, cheese, etc.)
Post-Introduction Assessment
Discussion Question: Ask students to suggest reasons why clean water is important. Write their reasons on the board. (Example answers: Water is a necessity of life; it provides food, entertainment, recreation; many people earn their living by commercial fishing, etc.)
Lesson Summary Assessment
Problem Solving: Addressing the entire class, ask students to calculate how much water they think the average person consumes in a lifetime. (Note: The water can be directly from drinking water or from other foods and liquids.) Require them to describe the amount in units of 8-ounce glasses, gallons, swimming pools, etc.
- Calculate how many 8-ounce glasses of water the average person consumes from food and liquid each year. (Answer: 8 glasses/day X 365 days/year = 2,920 glasses/year.)
- How many years does the average person live? (Answer: 75 years)
- Calculate how many 8-ounce glasses of water the average person consumes in a lifetime. (Answer: Assuming an average lifespan of 75 years, 75 years/life X 2,920 glasses/year = 219,000 glasses/lifetime.)
- To help students better understand this calculated amount, ask them to complete the How Much? Worksheet in small groups. Note: Each calculation addresses a different skill level; consider the skill level of your students before arranging groups for this activity. Discuss the results of the group calculations.
Homework
Take-Home Quiz: Administer the Water Pollution Quiz as a homework assignment. During the next class period, review the answers as a class.
Lesson Extension Activities
- Investigate factories in your community. Inquire about taking a tour of an industrial facility. What do they make? What types of water waste do they produce? How do they dispose of the waste? Research the company on the U.S. EPA website at https://www.epa.gov/.
- Invite a representative from the local wastewater or drinking water treatment plant to visit the classroom. Or, plan a field trip to the plant.
- Invite a local farmer or agricultural engineer into the classroom to talk about water-friendly farming practices.
- Ask students to locate the water meter in their home. Have them read the meter one night and then again at the same time a week later. Compare the results between readings of the same household and between students in class. Determine how much water per person is being used in each household. Are some households more water efficient? What are the reasons for this?
- Read the water meter at your home or school at the same time everyday for five days. Record and graph the data. Discuss ways to save water at home or school. Create a bulletin board display in your school to urge others to make similar changes.
- Contact the U.S. Bureau of Reclamation to inquire about their educational programs. They often have wonderful, hands-on demonstrations that they can bring to your class for little or no fee.
- Read "Rachel Carson and Shirley Briggs – Friends for Life" (from Environmental Portraits – People Making a Difference for the Environment, by Kim Sakamoto Steidl, Good Apple, Inc., 1993). Research and discuss the book Silent Spring by Rachel Carson (Houghton Mifflin Company, 1962). What impact did this information have on our current water pollution laws and practices?
- Assign students to complete the PCBs Worksheet and Desalination Handout to develop content area reading comprehension skills.
- Investigate the U.S. Geological Survey's Water Science for Schools website at: https://www.usgs.gov/special-topic/water-science-school
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students explore an important role of environmental engineers—cleaning the environment. They learn details about the Exxon Valdez oil spill, which was one of the most publicized and studied human-caused environmental tragedies in history.

Students learn about a special branch of engineering called bioremediation, which is the use of living organisms to aid in the clean-up of pollutant spills. Students learn all about bioremediation and see examples of its importance. In the associated activity, students conduct an experiment and see ...

Students are presented with examples of the types of problems that environmental engineers solve, specifically focusing on water quality issues. Topics include the importance of clean water, the scarcity of fresh water, tap water contamination sources, and ways environmental engineers treat contamin...

Students learn and discuss the advantages and disadvantages of renewable and non-renewable energy sources. They also learn about our nation's electric power grid and what it means for a residential home to be "off the grid."
References
Carson, Rachel. Silent Spring. New York, NY: Houghton Mifflin Company, 1962.
Glencoe Science: An Introduction to the Life, Earth and Physical Sciences, Student Edition. Blacklick, OH: Glencoe/McGraw-Hill, 2002.
Hopkins, Jean, Johnson, Susan and McLaughlin, Charles William. "How Many Cans of Soda Pop?" Ecology Earth's Natural Resources Activity Book, NJ: Prentice Hall, Inc., 1993. (ISBN 0-13-987090-3)
Kerrod, Robin and Evans, Ted. The Environment (Let's Investigate Science). New York, NY: Benchmark Books, 1993.
Lucas, Eileen. Water: A Resource in Crisis. Chicago, IL: Childrens Press, Inc., 1991.
Sakamoto Steidl, Kim. Environmental Portraits – People Making a Difference for the Environment. Boulder, CO: Good Apple, Inc., 1993.
Stille, Darlene R. The New True Book – Water Pollution. Chicago, IL: Childrens Press, Inc., 1990.
The National Academy of Engineer's ranking of the 20 best engineering achievements of the 20th century: http://www.greatachievements.org/
U.S. Environmental Protection Agency: http://www.epa.gov/owow/nps/qa.html
U.S. Geological Survey: https://www.usgs.gov/science/science-explorer/Water
Wisconsin Department of Natural Resources: http://dnr.wi.gov/org/caer/ce/eek/earth/recycle/index.htm
Product Stewardship Institute. Household Hazardous Waste. 2012, cdn.ymaws.com/www.productstewardship.us/resource/resmgr/imported/HHW%20Curriculum.pdf.
Copyright
© 2005 by Regents of the University of ColoradoContributors
Amy Kolenbrander; Jessica Todd; Malinda Schaefer Zarske; Janet YowellSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under grants from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation (GK-12 grant no. 0338326). However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: January 14, 2026






User Comments & Tips