Summary
Using a household fan, cardboard box and paper towels, student teams design and build their own evaporative cooler prototype devices. They learn about the process that cools water during the evaporation of water. They make calculations to determine a room's cooling load, and thus determine the swamp cooler size. This activity adds to students' understanding of the behind-the-scenes mechanical devices that condition and move air within homes and buildings for human health and comfort.
Engineering Connection
Engineers design heating, ventilation and air conditioning systems to provide thermal comfort for people who spend time in buildings and homes, all of which require energy to operate. Engineers are also challenged to provide optimum conditions for equipment, such as year round cooling loads to counteract the heat emitted from computers and electronic devices. To minimize the amount of energy spent powering these systems, engineers strive for high-efficiency and/or alternative approaches that use less energy to accomplish the same objective. Swamp coolers, or evaporative coolers, provide an alternative to typical air-conditioning, using up to 75% less energy to operate because they only require the operation of a fan and circulating pump. Swamp coolers are effective in dry climates with a low relative humidity, but are less effective in climates with a higher relative humidity.
Learning Objectives
After this activity, students should be able to:
- Explain the process that cools air during the evaporation of water.
- Calculate a room's cooling load (with a swamp cooling system).
- Explain how the engineering concepts in this design project can be applied to solve a real-world problem.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ESS3-4. Evaluate or refine a technological solution that reduces impacts of human activities on natural systems. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Design or refine a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: Analyze data using tools, technologies, and/or models (e.g., computational, mathematical) in order to make valid and reliable scientific claims or determine an optimal design solution.Alignment agreement: Use mathematical representations of phenomena or design solutions to describe and/or support claims and/or explanations.Alignment agreement: | Scientists and engineers can make major contributions by developing technologies that produce less pollution and waste and that preclude ecosystem degradation. Alignment agreement: When evaluating solutions it is important to take into account a range of constraints including cost, safety, reliability and aesthetics and to consider social, cultural and environmental impacts.Alignment agreement: | Engineers continuously modify these technological systems by applying scientific knowledge and engineering design practices to increase benefits while decreasing costs and risks. Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-2. Design a solution to a complex real-world problem by breaking it down into smaller, more manageable problems that can be solved through engineering. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Design a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | Criteria may need to be broken down into simpler ones that can be approached systematically, and decisions about the priority of certain criteria over others (trade-offs) may be needed. Alignment agreement: | Models (e.g., physical, mathematical, computer models) can be used to simulate systems and interactions—including energy, matter, and information flows—within and between systems at different scales. Alignment agreement: |
Common Core State Standards - Math
-
Model with mathematics.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Solve linear equations and inequalities in one variable, including equations with coefficients represented by letters.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Solve equations and inequalities in one variable
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Summarize, represent, and interpret data on a single count or measurement variable
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Rearrange formulas to highlight a quantity of interest, using the same reasoning as in solving equations.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the attributes of design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of engineering design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
A prototype is a working model used to test a design concept by making actual observations and necessary adjustments.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Evaluate ways that technology can impact individuals, society, and the environment.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Colorado - Math
-
Solve linear equations and inequalities in one variable, including equations with coefficients represented by letters.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Use units as a way to understand problems and to guide the solution of multi-step problems.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Rearrange formulas to highlight a quantity of interest, using the same reasoning as in solving equations.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Summarize, represent, and interpret data on a single count or measurement variable.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Solve equations and inequalities in one variable.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Reason quantitatively and use units to solve problems.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Colorado - Science
-
Gather, analyze and interpret data on chemical and physical properties of elements such as density, melting point, boiling point, and conductivity
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Use direct and indirect evidence to develop and support claims about the conservation of energy in a variety of systems, including transformations to heat
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- cardboard box (to fit around a small electric fan)
- paper towels, three-layers thick to cover box bottom
- waterproof glue, such as hot glue, epoxy or gorilla glue or packing tape
- Cooling Load Analysis and Computation Worksheet, one per person
- Psychrometric Chart, one per person
For the entire class to share:
- pencil
- scissors, to cut cardboard
- stapler
- compass, to mark curved lines to cut in the cardboard
- small electric fans (can be shared by several groups)
- adjustable water spray bottles
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_housing_lesson01_activity2] to print or download.Pre-Req Knowledge
Students should have a basic understanding of the concept of evaporation, as discussed in the associated lesson. Students should concurrently be taking Algebra 1 in order to complete the worksheet calculations.
Introduction/Motivation
Have you ever wondered why a fan can cool you down without lowering the temperature of the air blowing through it? Or, when you add a water misting device to the fan, why it cools you down even more? This can all be explained by the concept of evaporative cooling.
Consider the way our bodies regulate comfortable temperatures. We tend to shiver when we are cold, and sweat when we are too hot. When air is forced to quickly pass by our bodies, the moisture in our skin evaporates, taking heat with it, and cooling us down. The more moisture that evaporates, the faster we are able to cool ourselves, which explains why we sweat a lot when our bodies heat up. This concept of evaporative cooling is important to the thermal comfort of our bodies, and engineers use the same idea to create comfortable conditions within buildings and homes.
As early as the 1920s, people in dry, desert areas used the simple and effective evaporative cooling techniques to cool rooms and make them feel comfortable enough for sleeping. They would often sleep outside on porches surrounded by screens. Before going to bed, they soaked a few sheets in water and draped them over the screens. Then they used fans placed in the porch to pull air in from the outside, causing the air (through the evaporation of water) to cool as it passed through the soaked sheets. This same concept is applied today in what are commonly called "swamp coolers."
What better way to cut down on the cost of cooling your house than to use a swamp cooler! Since evaporative coolers only require a little bit of electricity to operate a fan and a pump, they consume up to 75% less energy than a typical (refrigeration-type) air conditioner, which can save hundreds of dollars per year. Of course, this kind of system has its limitations; it works best in a dry climate with hot summers. And, the equipment also costs less than half of what an air conditioner unit costs, due to the simplicity of its design. With all this in mind, today we're going to build our own swamp cooler prototypes.
Procedure
Background
How evaporation and evaporative cooling works: Evaporation is a natural process that is constantly happening all around us. For example, when our bodies heat up because of activity or hot weather, we release sweat that evaporates, helping our bodies cool. Another example of evaporative cooling can be seen near waterfalls, rivers, lakes and oceans. When dry air passes over bodies of water, some water evaporates, causing the relative humidity to rise. Liquid water molecules become vapor in the dry air through a process that uses energy to change its physical state. Heat is transferred through convection from the higher temperature of the air, to the lower temperature of the water, resulting in cooled air. The heat transferred to the water is consumed by the process of evaporation, so the water stays at a relatively constant temperature. Once the air becomes saturated, it can no longer hold more water, and evaporation stops. For this reason, water in high-humidity environment does not evaporate as quickly as that in a low-humidity climate, and hence swamp coolers are much more effective in dry climates.
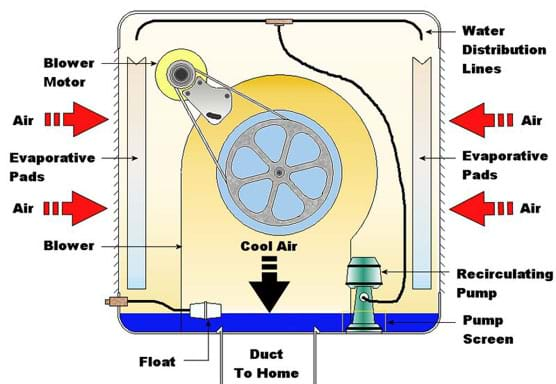
The reason we feel much warmer in a climate with high relative humidity is because we are not able to cool our bodies by sweating nearly as much. The high level of humidity prevents effective evaporation because the air is not able to take up much more vapor than it already holds. For the same reason, swamp coolers cannot work effectively in climates with high relative humidity because they depend on the evaporation of water to cool air. They also introduce more humidity into the air, which is undesirable in a climate that is already quite moist.
Difference between a swamp (evaporative) cooler and an air conditioner: The main difference between a swamp cooler and an air conditioner is that a swamp cooler uses evaporative cooling, while an air conditioner uses a heat pump and the vapor-compression refrigeration cycle. Swamp coolers have a simpler design, involving only a fan that pulls air through a medium saturated with water, raising the humidity and lowering the temperature of the air. An air conditioner uses a heat pump to circulate a liquid and transfer heat from a cooler place (the indoors) to a warmer place (outdoors). This vapor-compression refrigeration cycle is the same way that kitchen refrigerators operate. With an air conditioner, heat is transferred out of the indoor air without affecting its humidity at all, making it more desirable to use in humid climates. Air conditioners also require much more power to run the cycle.
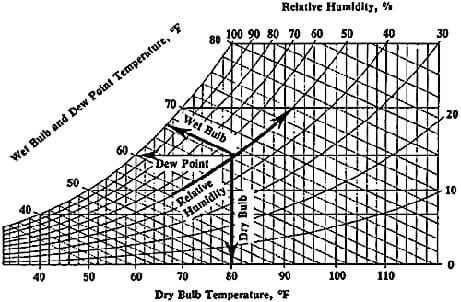
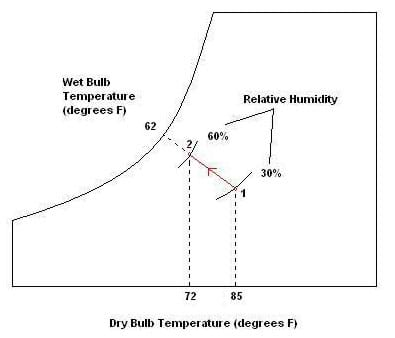
Psychrometrics: The way we analyze the conditions of water-vapor mixtures is through the use of a psychrometric chart (see attached, and Figure 1). With known dry bulb and wet bulb air temperatures, we can determine important information such as the percent relative humidity, and the dew point temperature. Using values from the chart, engineers analyze the conditions of air confined within a building or home, and use HVAC systems to regulate these values to a comfortable level. We may also use the values to estimate the effectiveness of a particular system. For example, the greater the difference in wet bulb and dry bulb temperatures, the lower the relative humidity, resulting in conditions more suitable for the use of a swamp cooler.
How to use a psychrometric chart: To use the chart, we must be given two values to determine our point of measure. For example, if given wet bulb and dry bulb temperatures, we can find the point of measure by observing where they line up. From here, we can read directly to the left to determine the dew point temperature, and read the curved lines to determine relative humidity (see Figure 1). We may also look up values such as humidity ratio, specific volume of air, and enthalpy, although for the purposes of this activity these are not useful. Note that along the curved line farthest to the left, the relative humidity is 100%, and the dry bulb and wet bulb temperatures are equal. As these temperatures differ more and more, the relative humidity decreases.
Cooling load calculation for swamp coolers: To calculate the cooling load of a room, we use a method that helps determine the correct size cooler. Swamp coolers are rated by CFMs, or cubic feet of air flow per minute. To keep a room cool, swamp coolers must displace the entire volume of a room approximately every two minutes. So, to determine the cooling load of a room, divide its volume (in cubic feet) by two minutes.
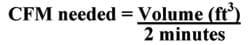
Example: For a 200 sq. ft. room, with 9-foot ceilings, the CFM rating needed would be.
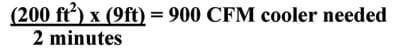
Before the Activity
- Gather materials.
- Make copies of the Cooling Load Analysis and Computation Worksheet and Psychrometric Chart (best printed in color), one each per student.
With the Students
- Divide the class into groups of two or three students each.
- Hand out worksheets and charts, one each per student.
- Once students have completed the first two parts of the worksheet, discuss their answers as a class to make sure the work was done correctly.
- Guide students through the next steps to design/build their own swamp (evaporative) cooler prototypes: Cut out the bottom of your cardboard box, leaving a rim (about 1-inch [2.5 cm] thick) around the edge. This becomes the back of the cooler. See Figure 2.

- Tear off three sheets of paper towels large enough to span the box floor. Staple them together to create one thick paper towel sheet.
- Glue or staple the paper towel sheet to the inside rim of the box floor (see Figure 3).
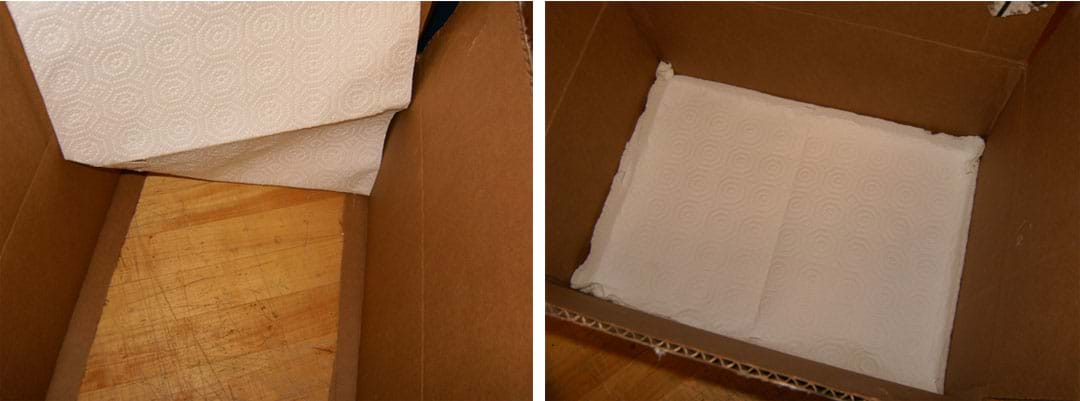
- Insert a small electric fan into the box with the front of the fan facing the top of the box. Then stand the box on its side. See Figure 4.
- Use a pencil to mark cut lines on the box flaps to make it clear what cardboard to remove from the front of the box so as to not block air flow to/from the fan. For round fans, use a compass to draw cut lines that result in a neat circle shape (see Figure 4).

- Cut out the marked areas on the box flaps (see Figure 5).
- Tape or glue the flaps down so that they form a seal around the fan. Use packing tape to seal up any other gaps in the cardboard casing so that air may only enter from the back (see Figure 5).

- Cut a flap on the top or the side of the box to permit access to the fan control (see Figure 6). Make sure you are able to close the flap again.

- Test the swamp coolers: On a hot day, have teams operate and test their swamp coolers to see if they can feel a temperature difference in air coming out from device. Have students feel the air coming out of the fan before and after they saturate the paper towels to see if they notice a difference.
- As students test and analyze their prototype devices, have them answer the worksheet questions and problems, completing their worksheets.
- Once testing and worksheet problems are competed, discuss results as a class.
- Conclude by conducting the engineering re-design and problem-solving exercises as described in the Assessment section.
Vocabulary/Definitions
convection: The transfer of heat by circulation of a gas (such as air) or liquid (such as water).
cooling load: A calculation made by mechanical engineers to size a building's HVAC system to remove excess heat gains from conduction, convection and radiation of building sources such as solar gains through the envelope (exterior surfaces), lights, people, appliances/office equipment, and outside air for ventilation.
dew point temperature: The temperature at which moisture condenses on a surface.
dry bulb temperature: A measure of the temperature of ambient air as measured with a common thermometer.
evaporation: The process whereby atoms or molecules in a liquid state gain enough energy to become a gas or vapor.
evaporative cooling: A means of temperature reduction that operates on the principle that water absorbs latent heat from the surrounding air when it evaporates.
HVAC system: Acronym for "heating, ventilation and air conditioning," which are the engineering systems responsible for creating and maintain comfortable and safe indoor air temperature, humidity and quality conditions.
prototype: An initial, full-scale attempt or early model of a new product, design or creation. May be revised many times.
psychrometrics: The field of engineering concerned with the determination of physical and thermodynamic properties of gas-vapor mixtures. The most common area of interest is in mixtures of water vapor and air because of its application in heating, ventilating and air-conditioning.
relative humidity : The amount of water vapor contained within a sample of air compared to the maximum amount of water vapor the air can hold
swamp cooler: A device that cools air by the evaporation of water into air. Also called evaporative coolers. The name "swamp cooler" comes from the humid air conditions produced.
thermal comfort: A state in which people are satisfied with their surrounding environment in terms of temperature, humidity and air movement. This concept recognizes that the sensation of feeling hot or cold is not just dependent on air temperature alone.
wet bulb temperature: A measure of the temperature of the wet thermometer bulb in a wet and dry bulb thermometer. It is defined as the lowest temperature to which air can be cooled solely by the addition of water. Measured with a thermometer with a wet sock over it, after being spun around allowing evaporation to occur.
Assessment
Pre-Activity Assessment
Discussion: Solicit, integrate and summarize student responses.
- Have you ever stood in front of a fan on a hot day to cool down? How does a fan cool down a room or a person without lowering the temperature of the air blowing through it?
Activity Embedded Assessment
Worksheet: Have students complete the activity worksheet; review their answers to gauge their mastery of the subject.
Question/Answer: Ask the students and discuss as a class:
- Why is it important to completely close off all parts of the device except the outflow? (Answer: To work, we want the device to pull air through the saturated water filter. If the fan were able to pull air from elsewhere, the air would not get cooled by passing by the water.)
Post-Activity Assessment
Engineering Re-Design: Have students think about their swamp cooler prototype design. What worked? What didn't work? What could you do to improve your design to work over a larger area? Have students think about the trade-offs involved in their design. Then have them make a sketch or write a description about any design changes they would make.
Problem Solving: Have students complete the following two-part exercise:
Problem 1: Sketch a psychrometric chart and draw the path of air being cooled by a swamp cooler. Indicate the starting and ending points as well as a direction. Label the dry bulb and wet bulb temperatures, as well as the relative humidity at each state. Use the initial conditions of 30% relative humidity and a room temperature of 85o F. Assume we want to stop cooling when the room gets to be 72o F, and that the wet bulb temperature remains constant.
Problem 2: A car has been out in the cold all night, but as the sun comes up the ambient temperature begins to rise. Suppose the surroundings warm up more quickly than the car windows, whose surface temperature is measured to be about 50o F. Assume the relative humidity is 60% and the dry bulb temperature is 65o F. Would water begin to condense on the surface of the car? If so, at what point would it stop? (Answer: Yes, water would begin condensing because the glass surface is below the dew point temperature (≈52o F) of the surrounding air. The condensation would stop when the glass surface temperature exceeds the dew point temperature.)
Investigating Questions
What are the pros and cons of using this type of cooling system for your home? (Answer: Pros — Reduces energy consumption while providing comparable cooling to that of an air conditioner, provides a constant exchange of fresh air throughout the space. Cons — Does not work effectively in moist climates, consumes a considerable amount of water that may be of concern in places prone to drought.)
Troubleshooting Tips
If you have trouble getting significant air flow out of the device, try poking a few small holes in the paper towel layers on the back.
Activity Extensions
Have students test the effectiveness of their prototype devices in a smaller room by measuring the ambient room temperature before and after a length of operation.
Compare the psychrometric chart for 5,000-ft. elevation with one for sea level (available on the Internet).
Activity Scaling
- For younger students, bring in some handheld water fan sprayers (usually $3 or less), and use these to demonstrate the same concepts.
- For more advanced students, create a large scale swamp cooler using a commercial fan with a circulating pump. This can be done as a class or in separate groups depending on available supplies.
Additional Multimedia Support
Show students a great animation of air flow through a swamp cooler at California Energy Commission's Consumer Energy Center's website: http://www.consumerenergycenter.org/home/heating_cooling/evaporative.html
Obtain color psychrometric charts from the website of Coolerado Cool Tools by Coolerado LLC in Arvada, CO. According to the website: "Feel free to print and copy these charts at will; we only ask that you leave the Coolerado logo on them." Charts are available in letter or ledger sizes for sea level, 2500 feet, 5,000 feet and 7,500 feet in pdf format. Note: A zip file of the different psychrometric charts can be found at http://www.coolerado.com/products/material-resource-center/?tabgarb=tab6
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students explore heat transfer and energy efficiency using the context of energy efficient houses. They gain a solid understanding of the three types of heat transfer: radiation, convection and conduction, which are explained in detail and related to the real world.

Students are introduced to passive solar design for buildings — an approach that uses the sun's energy and the surrounding climate to provide natural heating and cooling. They learn about some of the disadvantages of conventional heating and cooling and how engineers incorporate passive solar design...
References
Dictionary.com. Lexico Publishing Group, LLC. Accessed April 14, 2008. (Source of some vocabulary definitions, with some adaptation) http://www.dictionary.com
Evaporative Cooler. Updated February 12, 2008. Wikipedia, The Free Encyclopedia, Wikimedia Foundation, Inc. Accessed April 14, 2008. http://en.wikipedia.org/w/index.php?title=Evaporative_cooler&oldid=190871058
Evaporative Cooler Tips. The Home Energy Saver. Excerpted from No-Regrets Remodeling by Home Energy magazine. Accessed April 14, 2008. http://hes.lbl.gov/hes/makingithappen/no_regrets/evaporativecooler.html
Evaporative Cooling: How an Evaporative Cooler Works. Consumer Energy Center, California Energy Commission. Accessed April 14, 2008. http://www.consumerenergycenter.org/home/heating_cooling/evaporative.html
Copyright
© 2007 by Regents of the University of Colorado.Contributors
Landon B. Gennetten; Lauren Cooper; Malinda Schaefer Zarske; Denise W. CarlsonSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: October 24, 2021





User Comments & Tips