Quick Look
Grade Level: 4 (3-5)
Time Required: 45 minutes
Expendable Cost/Group: US $0.75
Group Size: 1
Activity Dependency: None
Subject Areas: Physical Science
NGSS Performance Expectations:

| 3-PS2-3 |
Summary
Students engage in the science and engineering practice of asking questions as they use balloons to perform simple experiments to make sense of the phenomena of static electricity and charge polarization. As they attract and repel objects with their charged balloons, students explore the disciplinary core idea of electronic and magnetic forces and the crosscutting concept of cause and effect.
Engineering Connection
Engineers make everyone happy: Environmental and electrical engineers design industrial air filters (such as electrostatic precipitators) that remove harmful pollutants from factory air without slowing down the production of the plant. Implementation of this technology has resulted in cleaner emissions into the air we breathe, from power plants, steel mills and other manufacturing and industrial processes and factories. Engineers can do this because they study static electricity and understand what is happening at the very tiny level of electrical charges, which are too small to see.
Learning Objectives
After this activity, students should be able to:
- Describe how to charge an insulator (such as a balloon).
- Explain what physical change occurs when an object is charged.
- Recognize that charged objects can stick to neutral insulators.
- Recognize that engineers must understand the concept of static electricity
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
3-PS2-3. Ask questions to determine cause and effect relationships of electric or magnetic interactions between two objects not in contact with each other. (Grade 3) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Ask questions that can be investigated based on patterns such as cause and effect relationships. Alignment agreement: | Electric, and magnetic forces between a pair of objects do not require that the objects be in contact. The sizes of the forces in each situation depend on the properties of the objects and their distances apart and, for forces between two magnets, on their orientation relative to each other. Alignment agreement: | Cause and effect relationships are routinely identified, tested, and used to explain change. Alignment agreement: |
Common Core State Standards - Math
-
Make sense of problems and persevere in solving them.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Fluently add and subtract within 1000 using strategies and algorithms based on place value, properties of operations, and/or the relationship between addition and subtraction.
(Grade
3)
More Details
Do you agree with this alignment?
-
Represent and solve problems involving multiplication and division.
(Grade
3)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
The process of experimentation, which is common in science, can also be used to solve technological problems.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
State Standards
Colorado - Math
-
Represent and solve problems involving multiplication and division.
(Grade
3)
More Details
Do you agree with this alignment?
-
Fluently add and subtract within 1000 using strategies and algorithms based on place value, properties of operations, and/or the relationship between addition and subtraction.
(Grade
3)
More Details
Do you agree with this alignment?
-
Find the unknown in simple equations.
(Grade
4)
More Details
Do you agree with this alignment?
Colorado - Science
-
Show that electricity in circuits requires a complete loop through which current can pass
(Grade
4)
More Details
Do you agree with this alignment?
Materials List
Each student needs:
- 1 small balloon
- 1 Charge It! Math Worksheet (optional math component)
- 1 calculator (if completing math worksheet)
For the entire class to share:
- A sink with a water faucet
- A few pieces of wool (available at fabric stores)
- A few packs of unflavored gelatin (not gelatin dessert)
- 1-2 small bowls
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_electricity_lesson02_activity1] to print or download.Introduction/Motivation
Ask students: When was the last time you walked across the carpet in your socks, went to open the door to another room and "ouch" you felt a small shock? Have you ever pulled your clothes from the clothes dryer and heard them crackle? How are these two situations related? What is causing the shock you feel or the crackle you hear?
Explain to students that during this activity they will explore static electricity, the electrical phenomenon that is causing all of these "mysterious" things to happen.
Before starting the activity, remind students that an atom consists of negatively-charged electrons, positively-charged protons and neutral-charged (neither positive nor negative charged) neutrons. Also, remind them that a positive and negative charge pull toward each other, positive charges repel other positive charges, and negative charges push away other negative charges. Remind students that static electricity occurs when there is an imbalance of positive charges and negative charges in an atom or object.
Next, tell students that scientists, environmental engineers and electrical engineers use their understanding of static electricity to develop industrial air filters to help our environment. These "electrostatic precipitators" use static electricity to remove pollutants without impeding the production efficiency of industrial plants (previous types of filters greatly reduced production efficiency, which was costly for the plants). Power plants, steel mills and paper plants use electrostatic precipitators to remove the harmful particles generated in the manufacturing process before they can pollute our air.
In the home, electrostatic air cleaners use an electrostatic force to move air molecules and trap small airborne particles (.05 – 30 microns in size, such as pet dander or other allergens) as they circulate past an array of electrically-charged stainless steel blades. One example is the Ionic Breeze by Zenion Effect Technology. Other technologies that exploit the properties of static electricity may be found in appliances and machines such as copy machines and printers designed by electrical and mechanical engineers.
Procedure
Background
How is it possible that you can rub a balloon on your hair or sweater and then stick the balloon to a wall? You may think that a charged object is only attracted to an oppositely-charged object, but a charged object can also be attracted to a neutral insulator. A charged object and a neutral insulator are attracted when the charged object causes a temporary reorientation of the charge in the atoms of the neutral insulator. This is commonly known as static charge, however at the atomic level this phenomenon is known as charge polarization.
In a typical atom, the positive charge (due to the protons) and the negative charge (due to the electrons) are evenly distributed around a center point. If we bring a negative charge close to this atom, the electrons in the atom are repelled and move away from the negative charge while still surrounding the nucleus of the atom. This makes one side of the atom more positively charged and the other side more negatively charged. The opposite situation occurs if we use an external positive charge.
This process of charge polarization also occurs in molecules. If we bring a negatively-charged balloon near a neutral insulator, such as a piece of wood, the electrons in the molecules of the wood move away from the negative balloon (while staying within their original wood molecules). This leaves the side of each wood molecule nearest the balloon more positively charged. As the charges align in the wood molecules, it produces a positively-charged area on the surface of the wood near the negatively-charged balloon. As a result, the balloon and wood temporarily "stick" to each other due to electrical attraction. Once the balloon is removed, the wood molecules' charge distribution returns to its normal state.
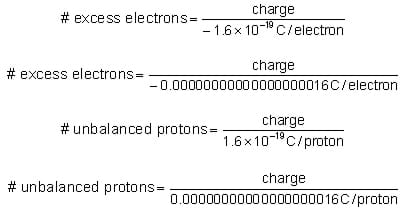
The coulomb [C] is the SI unit of charge. (SI is the abbreviation for the International System of measurement from the French Système Internationale.) One coulomb is equal to the amount of charge on 6.24 quintillion (6.24 1018 = 6,240,000,000,000,000,000) protons. Therefore, the charge in coulombs on one electron or proton is very, very small. To get a sense of how small the charge on one proton is compared to a coulomb, imagine counting protons. Say we count the first proton in one second, and we kept counting one proton every second until we had counted enough to have one coulomb of charge; it would take almost 198 billion years to count that number of protons!
The charge on an electron is –0.000 000 000 000 000 000 16 C. The charge on a proton is +0.000 000 000 000 000 000 16 C. It is more convenient to write very small and very big numbers using scientific notation. In scientific notation, the charge on an electron is –1.6 10-19 C and the charge on a proton is +1.6 10-19 C.
To determine the number of excess electrons or unbalanced protons, given the charge on an object, use the equations below. Exercises using these relationships are provided on the Charge It! Math Worksheet.
Before the Activity
- Cut out a few squares of wool.
- Place the contents of a few packs of gelatin in plastic bowls.
- Make copies of the Charge It! Math Worksheet.
With the Students
- Ask students: True or false: Are electrons the only particles in atoms that can move between materials? (Answer: True.) True or false: Do like-charged objects attract while unlike-charged objects repel? (Answer: False. The reverse is true.)
- Have students blow up their balloons and tie knots in the ends.
- To charge the balloons, have students rub their balloons vigorously on their hair or on a piece of wool.
- Have students try each of the following and write their observations on a sheet of paper or in their science journal. Remind them to recharge the balloon between each experimental trial.
- Move a finger toward the balloon.
- Hold two charged balloons near each other.
- Put the balloon on a large tabletop (or the floor) and try to gently roll it.
- Try to stick the balloon to one or more of these surfaces (or any others around the classroom): wooden door, wall, metal desk, metal file cabinet, blackboard, window, plastic chair, clothing, mirror.
- Have a few students stick their balloons to different surfaces and leave them there. Have one or two students time how long each balloon stays up.
- Hold a balloon near a thin, steady stream of water (see Figure 1). Try a stream of water about an eighth of an inch in diameter.
- Hold the balloon above a bowl of gelatin (see Figure 2).
- For related math practice, have the students use calculators to complete the Charge It! Math Worksheet.

Activity Observations and Explanations
A balloon rubbed on clothing becomes negatively charged because the balloon is made from an insulating material (usually natural latex from rubber trees). Electrons deposited on the balloon are confined to the region that was rubbed, so only a portion of the surface of the balloon is negatively charged.
When you hold your finger near a charged balloon you hear a crackling sound due to the balloon discharging. The spark is too small and fast to see with the human eye. The same thing happens if you hold two charged balloons near each other.
When you try to roll a charged balloon, you notice that the balloon only rolls a short distance; then, it stops and wobbles about the portion of the balloon that you charged. This charged portion of the balloon "sticks" to the floor (a neutral insulator) due to charge polarization that occurs in the molecules of the floor.
A charged balloon "sticks" to a wooden door, a wall, ceiling, a plastic chair, window, mirror, and clothing due to charge polarization in these insulators. It quickly slides down a metal surface because metal is a good conductor and electrons "leak off" the balloon to the metal.
A charged balloon deflects or attracts a stream of water because water is a polar molecule. Water molecules have an uneven charge distribution such that the oxygen end is more negative and the hydrogen ends are more positive. When the negatively-charged balloon is brought near the stream of water, the negative ends of the water molecules are repelled by the balloon. If the water molecules spin around so their negative ends point away from the negative balloon, the attraction between the positive ends of the water molecules and the negative balloon causes the water stream to move toward the balloon.
When you hold a balloon over a bowl of gelatin, small stalactites of gelatin form and hang from the balloon surface. This attraction is explained by the polar nature of the gelatin molecules. The positive ends of the gelatin molecules are attracted to the negative balloon. The gelatin molecules form a chain because the positive end of each molecule is attracted to the negative end of another molecule. (However, it may also occur because the gelatin molecules are hydrated, meaning that each gelatin is attached to a water molecule. If this is the case, the positive ends of hydrated molecules form a chain.)
Some of you may have seen anti-cling or anti-static products that you place in the dryer with your clothes or spray onto your clothes if they stick together due to static electricity. How do you think they work? The dryer sheets work by reducing the friction between the clothes rubbing together. Each dryer sheet has a waxy substance on it that is transferred to your clothing in the dryer and reduces static electricity. The anti-static sprays work the same way by adding a waxy substance or lubricant to the fibers of your clothes.
Assessment
Pre-Activity Assessment
Voting: Ask a true/false question and have students vote by holding thumbs up for true and thumbs down for false. Tally the votes and write the totals on the board. Give the correct answers. Ask the students:
- True or False: Electrons are the only particles in atoms that can move between materials. (Answer: True.)
- True or False: Like-charged objects attract while unlike-charged objects repel. (Answer: False. The reverse is true.)
Activity Embedded Assessment
Question/Answer: Ask students questions and discuss as a class:
- What happens to the balloon when you rub it with wool? (Answer: It becomes charged.)
- What happens to the charge over time? (Answer: It leaks off the balloon.)
- What is the negatively-charged part of an atom called? (Answer: An electron.)
Post-Activity Assessment
Student Pairs: Have students work in teams of two on the following questions. First, each student should answer each question and then they should confer on the answers. If there is agreement between them, they raise their hands for the teacher to check their answers. If they disagree on the answers, they may confer with another team.
- Do like charges repel or attract one another? (Answer: Repel.)
- Why do two materials become charged when they are rubbed together? (Answer: Electrons are rubbed off one object onto the other.)
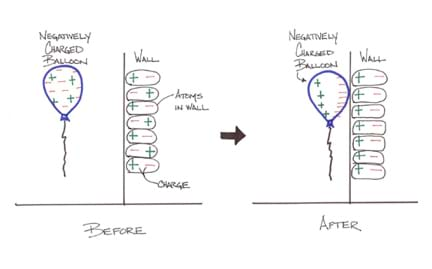
- Why does the negatively-charged balloon stick to the wall even though the wall is electrically neutral? (Answer: Electrons in the particles in the wall move away from the negative balloon so the sides of the particles toward the balloon are more positively charged. See Figure 3)
Drawing: Have students depict their subject knowledge gained by drawing a picture. Have them sketch and label some of the concepts or activities. For example, draw a sketch of what happens to a wall when you touch a negatively-charged balloon to it. (Possible answer: A drawing would show the balloon sticking to the wall with electrical charges, as indicated in Figure 3.)
Investigating Questions
- What are the causes and effects of static electricity?
Safety Issues
- Ask students not to hold inflated balloons next to their faces or other students' faces, in case the balloons pop.
Troubleshooting Tips
Remind students to recharge the balloon between each experimental trial. Students sometimes unintentionally hold or touch the part of balloon that they charged, thus discharging the balloon. If they do this, they might not be able to "attach" it to another surface.
A charged balloon does not stick to surfaces very long in humid weather because the presence of water facilitates the removal of electrons from the balloon to the surface more quickly than the electrons naturally disperse on a dry surface. This demonstrates that water is a good conductor while dry air is a good insulator.
Activity Extensions
Ask students to brainstorm how to measure the flow of water that is being deflected by the charged balloon. Possible ideas: using a ruler or protractor to measure the angle, etc.
Cut tissue paper into small pieces. Using a charged balloon, have the students make a chain of tissue paper squares. Charge polarization in the tissue paper allows the paper to be attracted to a charged balloon and to each other, thus allowing a chain to form. See if students can correlate the number of tissue paper pieces in the chain with the amount of time or number of rubs used in charging the balloon. Have the students graph their results.
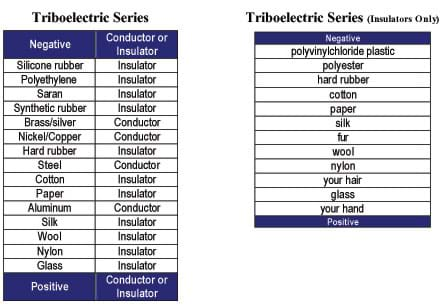
It can be useful for students to see that many different types of materials can be charged. Students might be interested in charging other objects and determining their charge. Figure 4 provides a triboelectric series — a ranking of materials' ability to hold or give up electrons. The triboelectric series on the left consists of both conductors and insulators, while the series on the right consists of only insulators. If you rub a material that is closer to the negative end of the series with a material that is closer to the positive end of the series, the items will charge negatively and positively, respectively. Use the Triboelectric Series Reference Sheet as a classroom handout.
Activity Scaling
- For lower grades, the math worksheet may be too advanced for most students.
- For upper grades, conduct the activity as is, having students use calculators to complete the Charge It! Math Worksheet. For extra math, have the students determine if there is a relationship between the amount of time you charge the balloon and the amount of time it sticks to a surface. Have students make a line graph and a table of charge time vs. "hang" time. Ask students to make predictions for other charging times (i.e., if the chart has 10, 20 and 30 seconds of charge time, what might happen at 25 seconds and 40 seconds?).
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students come to understand static electricity by learning about the nature of electric charge, and different methods for charging objects. In a hands-on activity, students induce an electrical charge on various objects, and experiment with electrical repulsion and attraction.

This lesson introduces the concept of electricity by asking students to imagine what their life would be like without electricity. Students learn that electrons can move between atoms, leaving atoms in a charged state.

Students are introduced to the concept of electricity by identifying it as an unseen, but pervasive and important presence in their lives. They compare conductors and insulators based on their capabilities for electron flow. Then water and electrical systems are compared as an analogy to electrical ...

Students explore the static electricity through hands-on activities. They attract O-shaped cereal pieces to charged combs and watch the cereal jump away when it touches the comb. They also observe Styrofoam pellets pulling towards a charged comb, then leaping back to the table.
References
Electrical Engineering for Pollution Control, Electrostatic Precipitator for Power Plants, ASU Electrical Engineering. holbert.faculty.asu.edu/wise/electrostaticprecip.html Accessed February 2004.
Copyright
© 2004 by Regents of the University of Colorado.Contributors
Xochitl Zamora Thompson; Sabre Duren; Joe Friedrichsen; Daria Kotys-Schwartz; Malinda Schaefer Zarske; Denise W. CarlsonSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: January 28, 2021






User Comments & Tips