Quick Look
Grade Level: 12 (10-12)
Time Required: 30 minutes
Lesson Dependency:
Subject Areas: Chemistry, Physical Science, Physics
NGSS Performance Expectations:

| HS-PS2-6 |

Summary
Students are presented with the concepts of wetting and contact angle. They are also introduced to the distinction between hydrophobic and hydrophilic surfaces. Students observe how different surfaces are used to maintain visibility under different conditions.Engineering Connection
Chemical engineers understand that a liquid's tendency to either bead or sheet on a solid surface is determined by the properties of both the surface and the liquid. The resulting hydrophilic ("water loving") and hydrophobic ("water hating") surfaces both have important applications in all types of engineering, including chemical, automotive, nautical, industrial and civil engineering. Applying a hydrophilic anti-fog coating to glass causes any condensation to form into a thin, even layer of water instead of droplets, so the glass remains transparent. Adding hydrophobic rain-repellent glass treatments to windshields causes water to bead and roll off the windshield surface to improve visibility (and safety). Hydrophobic surfaces are also important in protecting surfaces from water damage and stains.
Learning Objectives
After this lesson, students should be able to:
- Define the term "wetting" and describe how total wetting, partial wetting and no wetting are different.
- Explain how water behaves differently when in contact with hydrophilic and hydrophobic surfaces and how this relates to total, partial and no wetting cases.
- Discuss situations in which applying a hydrophilic or hydrophobic surface would be advantageous.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS2-6. Communicate scientific and technical information about why the molecular-level structure is important in the functioning of designed materials. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Apply scientific principles and evidence to provide an explanation of phenomena and solve design problems, taking into account possible unanticipated effects. Alignment agreement: | Attraction and repulsion between electric charges at the atomic scale explain the structure, properties, and transformations of matter, as well as the contact forces between material objects. Alignment agreement: | The functions and properties of natural and designed objects and systems can be inferred from their overall structure, the way their components are shaped and used, and the molecular substructures of its various materials. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Chemical technologies provide a means for humans to alter or modify materials and to produce chemical products.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
North Carolina - Science
-
Compare physical and chemical properties of various types of matter.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Introduction/Motivation
(Show Figure 1 to the class, either by projection or by passing around a printout. While students are looking at it, ask them questions about previous surface tension lessons and activities, such as the following:)
- What makes water stick to itself and form bubbles and droplets? (Answer: Cohesive forces—the attraction of liquid particles to one another.)
- How does a water strider stay above water? (Answer: Surface tension creates a slight "skin" of more densely packed water molecules. By using a surface on its legs that repels water, the water strider can walk on this "skin" of water without breaking through.)
- Why do water droplets stick to things? (Answer: Adhesive forces—the attraction of liquid particles to other objects.)
- What causes water to be absorbed by a paper towel or move up a capillary tube? (Answer: Capillary action—a combination of cohesive and adhesive forces.)
Look closely at this photograph. What is happening to the rain on the windshield? In order to see better through the windshield, how would you rather the water acted? Do you want the water to stick to the surface or roll off of it? What could you do to make visibility better? (Students may have their own experiences driving in the rain; as time permits, let students share and discuss their experiences. Give students time to think about their answers and make their best educated guesses using what they know from personal experiences.)
Look at the photograph again. What is causing the window to fog up? How does this affect visibility? Would increasing or decreasing the attraction of the water for the glass improve visibility? What could you do to make visibility better?
What causes the "fogging" effect? Both atmospheric fog and "window" fog are made of tiny droplets of water, but they are not the same thing. In both cases, the tiny droplets scatter light in all directions rather than allowing light to pass straight through. This is why it is not helpful to use your high beams in a fog — because the light gets scattered back in a heavy fog when it wouldn't on a clear night. Have any of you experienced this kind of fogging? (Students may relate stories from driving, as well as glasses and goggles fogging up. You may want to remind students that condensation forms because of a temperature difference between a surface and air.)
The fog on the inside of a window is a comparatively small amount of water. An anti-fog coating causes the water to form a layer of water rather than droplets, resulting in a very thin layer of water that is easy to see through. Outside the car window, there is a lot more water to contend with. Even if you could apply a coating to the outside of the window that caused the water to form a layer of water rather than drops, the water layer would accumulate very quickly and the water sheet would soon break up and reduce visibility. In this case, then, it is much more effective to apply a coating that helps remove the water as quickly as possible.
That's where contact angles come into play. A contact angle is a liquid's tendency to either bead or sheet on a solid surface and is determined by the properties of both the surface and the liquid. A hydrophobic, or "water hating" surface, causes water to form droplets on the surface and easily leave the surface. Adding hydrophobic rain-repellent glass treatments to windshields causes water to bead and roll off the surface of the windshield and make driving less treacherous during rainy or snowy conditions.
By contrast, a hydrophilic, or "water loving" surface, causes water to spread out and cover a surface rather than bead. These types of surfaces are especially useful to avoid loss of visibility due to condensation. As we just discussed, water condensates on glass and causes it to become "foggy" because the tiny water droplets formed on the surface scatter light. When a hydrophilic anti-fog coating is applied to the glass, however, the condensation forms a thin, even layer of water instead of the droplets and the glass remains transparent.
Both of these types of surfaces have important applications in automotive and nautical engineering. These treatments may also be important to engineers when considering visibility through glass windows and partitions in buildings. Hydrophobic surfaces are also important in protecting surfaces from water damage and stains.
Lesson Background and Concepts for Teachers
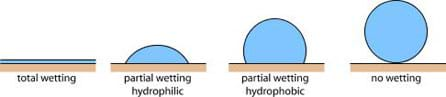
When a liquid drop is placed onto a solid surface, its behavior depends on the adhesive forces between the liquid and the surface (see Figure 2).
If the adhesive forces are attractive (the liquid is attracted to the solid surface), the liquid drop is pulled toward the surface and spreads along the surface. This type of surface is called "hydrophilic," meaning water loving. If the adhesive forces are repellent (the liquid is repelled by the solid surface), the liquid drop minimizes its contact with the surface and is said to "bead." This type of surface is called "hydrophobic."
The photographs in Figure 3 show examples of water on hydrophilic and hydrophobic surfaces. The surface on the left is hydrophilic, so the water droplets spread out to increase the area of the solid surface in which they are in contact. The surface on the right is hydrophobic, so the water beads up in order to decrease the area of the solid surface in which each bead is in contact.
Hydrophobic materials and coatings are used to make water-repellant clothing and backpacks. The inside of milk cartons is coated with wax, a hydrophobic material, to make them waterproof. Wax is also applied to cars and other vehicles to protect them from the weather. Chemical engineers incorporate hydrophobic elements into outdoor paints and stains to protect wood and other building materials from the elements.
Adding "wetting" agents to lower the contact angle and allow liquids to spread are also useful in many areas. Wetting agents increase the ability of paints and other coatings to spread and penetrate surfaces. They improve the surface contact, and therefore improve adhesion, of cements and glues. Anti-fogging coatings are added to camera lenses and diving masks so that condensation forms a thin, transparent sheet of water rather than many small droplets that impede vision.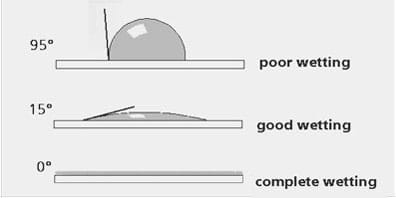
The strength of the attractive or repellant force is closely related to the "contact angle" between the water drop and the surface (see Figure 4). On a hydrophilic surface, the contact angle will be less than 90°; the water drop tends to spread out and "wet" the surface. On the other hand, if the surface is hydrophobic, the contact angle will be greater than 90°, and instead, the water drop tends to bead up on the surface.
Associated Activities
- Investigating Contact Angle - Students observe the behavior of water when placed on 3-5 different hydrophilic and hydrophobic surfaces. They determine which coatings are best to cause a surface to shed water quickly or to reduce the "fogging" caused by condensation.
Assessment
Pre-Lesson Assessment
Opening Questions: On a note card or in small groups, have students brainstorm to come up with plausible answers to the following questions. Ask for the most in-depth answers they can provide.
- Why does your mirror steam up when you take a shower? (Answer: As water condenses on the mirror, it forms many small droplets that scatter the light and "fog" the glass.)
- Why do some drivers wax their cars? (Answer: At least one reason is to repel water so that rain runs off the surface.)
Post-Introduction Assessment
Water Droplet ID: Find many photographs from Google Images or another source that show water droplets on various surfaces. By looking closely at the images, have students identify the drops as hydrophilic (total wetting or drops with small contact angles) or hydrophobic (no wetting or drops with large [> 90°] contact angles). As a class, discuss student answers. This activity helps to prepare students to identify the surfaces in the associated activity lab as hydrophilic or hydrophobic.
Lesson Summary Assessment
Three-Minute Writing: Hand each student a note card. Instruct them to write down — in three minutes — everything they learned in today's lesson. It doesn't matter if the information is out of order. Small diagrams or drawings are fine. Encourage students to try to fill the note card front and back in the time given. Require that they do this from memory — they may not refer to their notes.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students observe how water acts differently when placed on hydrophilic and hydrophobic surfaces. They determine which coatings are best to cause surfaces to shed water quickly or reduce the "fogging" caused by condensation.

Students are introduced to superhydrophobic surfaces and the "lotus effect." Students learn how plants create and use superhydrophobic surfaces in nature and how engineers have created human-made products that mimic the properties of these natural surfaces.

Students are presented with a short lesson on the difference between cohesive forces (the forces that hold water molecules together and create surface tension) and adhesive forces (the forces that causes water to "stick" to solid surfaces. Students are also introduced to examples of capillary action...

Students investigate the property dependence between liquid and solid interfaces and determine observable differences in how liquids react to different solid surfaces. They compare copper pennies and plastic "coins" as the two test surfaces.
References
de Gennes, Pierre-Gilles. "Wetting—Statics and Dynamics." Reviews of Modern Physics, American Physical Society. 57 (1985): 827-863. Accessed September 1, 2010. http://rmp.aps.org/abstract/RMP/v57/i3/p827_1
de Gennes, Pierre-Gilles, et al. "Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves." New York, NY: Springer, 2004.
Copyright
© 2013 by Regents of the University of Colorado; original © 2011 Duke UniversityContributors
Jean Stave, Durham Public Schools, NC; Chuan-Hua Chen, Mechanical Engineering and Material Science, Pratt School of Engineering, Duke UniversitySupporting Program
NSF CAREER Award and RET Program, Mechanical Engineering and Material Science, Pratt School of Engineering, Duke UniversityAcknowledgements
This digital library content was developed under an NSF CAREER Award (CBET- 08-46705) and an RET supplement (CBET-10-09869). However, these contents do not necessarily represent the policies of the National Science Foundation, and you should not assume endorsement by the federal government.
Special acknowledgements to Jonathan Boreyko, Mechanical Engineering and Material Science, Pratt School of Engineering, Duke University.
Last modified: June 13, 2021






User Comments & Tips