Quick Look
Grade Level: 6 (5-7)
Time Required: 4 hours 45 minutes
(seven 40-minute sessions)
Expendable Cost/Group: US $8.00
Group Size: 4
Activity Dependency: None
Subject Areas: Chemistry, Earth and Space, Physical Science, Problem Solving, Reasoning and Proof
NGSS Performance Expectations:

| 5-PS1-1 |
Summary
Shine a light on the fascinating world of chromatography! Students investigate different colored pigments in a variety of different colored leaves. By using isopropanol and chromatography paper, students separate the different pigments that make up the color of the leaf. They learn to analyze data by collecting and recording information after assembling an experiment in which they use the paper chromatography method. Students further learn about pigmentation by making sense of the process of the phenomena of photosynthesis. Students learn that producers (e.g., plants), have chlorophyll which absorbs the sunlight to produce the food they need to survive and the type of chlorophyll a plant has affects the color of the plant.
Engineering Connection
Chromatography is used in a variety of fields such as environmental, biochemical, and chemical engineering. Environmental engineers use chromatography to identify toxic substances in drinking water. Biochemical and chemical engineers use chromatography to examine different components that can be found in items such as: pharmaceuticals, food, and beverages. Microbiologists can analyze different bacteria in plants by utilizing chromatography.
Learning Objectives
After this activity, students should be able to:
- Describe what chromatography is and how the process works.
- Analyze that a leaf is a mixture of color pigments by assembling an experiment in which they will separate the colors using the paper chromatography method.
- Comprehend that the compound chlorophyll is located inside a plant’s cells and it absorbs the sunlight to start photosynthesis.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
5-PS1-1. Develop a model to describe that matter is made of particles too small to be seen. (Grade 5) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to describe phenomena. Alignment agreement: | Matter of any type can be subdivided into particles that are too small to see, but even then the matter still exists and can be detected by other means. A model showing that gases are made from matter particles that are too small to see and are moving freely around in space can explain many observations, including the inflation and shape of a balloon and the effects of air on larger particles or objects. Alignment agreement: | Natural objects exist from the very small to the immensely large. Alignment agreement: |
Common Core State Standards - English
-
Report on a topic or text, tell a story, or recount an experience in an organized manner, using appropriate facts and relevant, descriptive details to support main ideas or themes; speak clearly at an understandable pace.
(Grade
4)
More Details
Do you agree with this alignment?
-
Review the key ideas expressed and explain their own ideas and understanding in light of the discussion.
(Grade
4)
More Details
Do you agree with this alignment?
-
Conduct short research projects that use several sources to build knowledge through investigation of different aspects of a topic.
(Grade
5)
More Details
Do you agree with this alignment?
-
Produce clear and coherent writing in which the development and organization are appropriate to task, purpose, and audience.
(Grade
5)
More Details
Do you agree with this alignment?
-
Write informative/explanatory texts to examine a topic and convey ideas and information clearly.
(Grade
5)
More Details
Do you agree with this alignment?
-
Come to discussions prepared, having read or studied required material; explicitly draw on that preparation and other information known about the topic to explore ideas under discussion.
(Grade
5)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop abilities to assess the impact of products and systems.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Texas - Science
-
construct tables and graphs, using repeated trials and means, to organize data and identify patterns; and
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
recognize that radiant energy from the Sun is transformed into chemical energy through the process of photosynthesis;
(Grade
7)
More Details
Do you agree with this alignment?
Materials List
For Day 1: Introduction/Motivation
Each student needs:
- Day 1 Parent Letter
- Pre-Assessment
- science notebook for students to write down observations
Teacher needs:
- 2 purple skittles (any color can be used, but purple had better results than most colors
- clear glass straight sided jar or glass container, ~475 mL (16 oz.)
- ruler
- scissors
- piece of chromatography paper 50 mm x 125 mm (or coffee filter paper), examples available online: https://www.amazon.com/Whatman-3030-6189-Cellulose-Chromatography-Length/dp/B005FM4YYQ
- 6 mL isopropyl alcohol
- 10 mL graduated cylinder
- piece of 75 mm x 75 mm inches aluminum foil
- 1 mL dropper (plastic or glass)
- 3 mL pipette (or toothpick)
- pencil
- small binder clip
For Day 2: Outdoor Investigation
Each group needs:
- Outdoor Investigation Sheet (*optional, student can write it in their journal)
- Outdoor Investigation Rubric
- glue or tape (*optional; only needed if students will glue attachments into notebook)
- science notebooks for each student
- pencil for each student
For Day 3: Running Chromatography
Each group needs:
- Chromatography #1 Worksheet (*optional, student can write it in their journal)
- Exit Ticket (1 ticket per student)
- leaves collected by students
- 2 mortars and pestles (or wooden spoon and jar), 60 cm in diameter
- 2 clear glass straight sided jars or glass containers, ~475 mL (16 oz.)
- plastic wrap to cover the top of the jars
- 2 10-mL graduated cylinder
- container of 6-10 mL of isopropyl alcohol (or solvent of choice)
- 8 pieces of chromatography paper 50 mm x 125 mm (or coffee filters)
- 2 mini binder clips (or mini clothespins)
- safety goggles (1 per student)
- 2 rulers
For Day 4: Running Chromatography
Each group needs:
- Chromatography #2 Worksheet per student (*optional, student can write it in their journal)
- 3-5 leaves collected by the teacher for testing
- 2 mortars and pestles (or wooden spoon and jar), 60 cm in diameter
- 2 half-pint canning jar with lid (or glass cup with saran wrap to cover the top)
- 2 10 mL graduated cylinders
- container of 6-10 mL Isopropyl alcohol (or solvent of choice)
- 8 pieces of chromatography paper 50 mm x 125 mm (or coffee filters)
- Saran wrap
- 4 mini binder clips (2 per pair)
- goggles (1 per student)
- pair of safety gloves (1 per student)
- pencil (1 per student)
- ruler (2 per group)
For Day 5: Create Reflection Project (Post-assessment)
Each group needs:
- Check-in Ticket for each student
- Reflection Project Rubric for each student
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/uot-2460-fun-leaf-chromotography-activity] to print or download.Pre-Req Knowledge
Basic knowledge of mixtures and solutions, photosynthesis, and plants as producers in our ecosystem.
Introduction/Motivation
Has anyone heard of chromatography or have any inferences as to what chromatography might be? (Listen to students’ responses and jot them down on board. Possible student responses: taking a mixture and separating it into its different parts, using a solvent to look at different properties that make up a mixture.)
Yes, chromatography is the separation of a mixture into individual components. There are different kinds of chromatography that are used amongst many different scientists and engineers. For example, environmental engineers use chromatography to remove contaminants or toxic substances from drinking water. Biochemical and chemical engineers use chromatography to examine different components that can be found in items such as: pharmaceuticals, food, and even beverages. Microbiologists analyze different bacteria in plants by using chromatography. A very common type of method of chromatography that is used in organic chemistry labs with chemists, is called thin layer chromatography. Even forensic scientists use chromatography to analyze different mixtures to identify different components in a mixture. Because of this, chromatography has been used to solve many different crimes! By doing chromatography, scientists can inspect, analyze and identify different substances, from ink to objects on a much larger scale like explosive devices.
Chromatography is the separation of a mixture into individual components. Chromatography works with two phases: the stationary and mobile phase. The chromatography paper, or the stationary phase, absorbs the pigments while the mobile phase pulls the solvent up the paper. As the solvent moves up the paper it separates the mixture into its colors or components.
We are going to see how chromatography works with a purple skittle demonstration. The rest of this week, we will be exploring more about chromatography with leaves and you will be running the experiments in your own research groups to learn more about photosynthesis and the hidden colors or pigments in leaves!
Procedure
Background
Plants use light energy from the sun to produce the food they need to survive. This is called photosynthesis. The light from the sun is absorbed by the plant’s cells. Inside some of these cells are compounds called chlorophyll. Chlorophyll absorbs the sunlight to start photosynthesis during warm seasons. Photosynthesis is the process in which a plant uses light energy from the sun to produce food they need to survive.
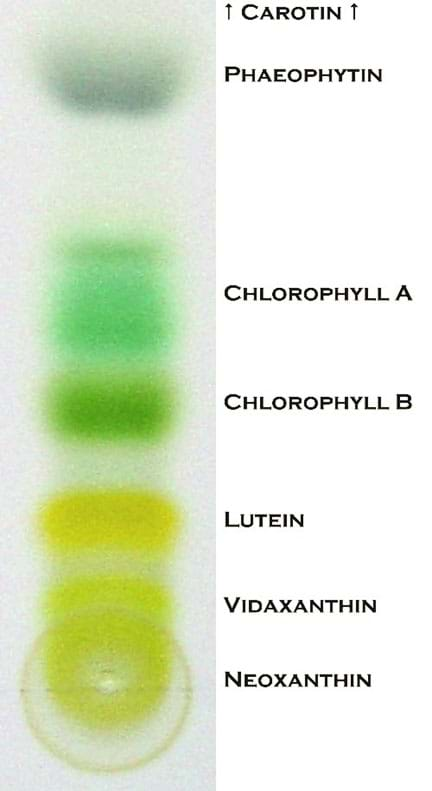
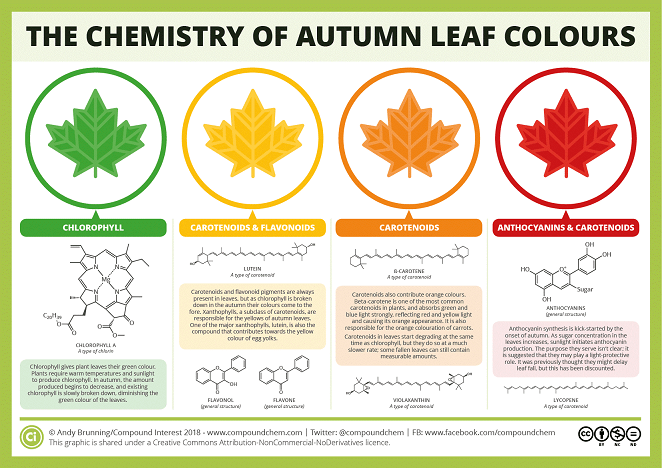
Often when we look at plants and leaves they tend to mostly be green. This is because the leaves have more chlorophyll than any other pigments. Chlorophyll is green because the light it absorbs is in the blue and red spectrum. Green light is not absorbed, but rather reflected, making chlorophyll appear green. However, in the fall they begin to change color, this is because chlorophyll breaks down, which causes the other pigments that have been there, start to appear. Chlorophyll breaks down due to the leaves stopping photosynthesis (their food making process) during the fall because of the change in daylight and changes in temperature. Some of the other pigments that begin to appear include compounds such as: carotenoids, xanthophylls, and anthocyanin. Carotenoids are the compounds that create the orange pigment in leaves. Xanthophyll is a type of carotenoid; it has a compound called lutein that creates the yellow pigment in leaves. There is also red pigment in the leaves that comes from the compound anthocyanin.
To see these hidden colors, we can do chromatography. When we use chromatography we have the stationary phase (chromatography paper) and a mobile phase (isopropanol) that helps move the different components of the mixture up the paper. The different chemical compounds will travel up the paper at different rates depending on their solubility. If a substance (A) is very soluble (meaning that it can be dissolved in a solvent more than other compounds) it will travel farther up the paper and if a compound (B) is less soluble (meaning that it is harder to be dissolved in a solvent) it won’t travel as far up the paper.
Video on Explanation of the Basics of Paper Chromatography and Thin Layer Chromatography https://vimeo.com/182048032.
Before the Activity
- Before the outdoor activity send home the note (Day 1 Parent Letter) to students’ parents requesting each student to collect 3-5 leaves of 2-3 different trees from their neighborhood/community.
Teacher obtains 3-5 leaves for each group from 2-3 of the plants in the following list. These plants are recommended because they show a different color on the chromatography paper.
- Ginkgo (when it is green) will show yellow on paper.
- Tradescantia pallida, “Purple Heart” (when it is purple) will show some green and brown after running chromatography.
- Heavenly Bamboo Gulf Stream (when it is mostly red) will show some yellow
Day 1 (40 minutes): Engage/Pre-assessment
- Give the students the Pre-Assessment.
- Read the Introduction/Motivation section to the class.
Teacher instructions for demo:
- With a pencil, take the chromatography paper and write the date and “purple skittle” at the top.
- Using a ruler, measure 1 cm up from the bottom of the paper. Draw a horizontal line from one end of the paper to the other end 1 cm from the bottom. In the middle of the line draw a tiny X; this will be the starting point.
- Next take a piece of aluminum foil and with the dropper add 4 drops of water onto the foil.
- Put the purple skittle on the piece of foil and wait until the color of the water changes.
- With the pipette tip, dip it into the skittle dye. Then dab it on top of the X on the chromatography paper.
- Continue dabbing dots onto the chromatography paper five more times, but make sure to let them dry before adding more.
- Pour 6 mL of isopropanol into the jar.
- Place the chromatography paper into the jar (making sure the solvent does not pass the 1 cm line and that the paper does not slouch in the jar). A binder clip can be used to support the paper and from keep it from sliding into the jar. Be sure to clip it securely to the top of the jar.
- Have the students observe what happens as the solvent pulls up the color pigments of the skittle.
- Students should write down their observations and what they think happened. (Students should observe: the chromatography paper [the stationary phase] absorbing the pigments and the solvent moving up the paper, creating the color of the skittle to look as if it is spreading up the chromatography paper.)
- Have the students explain in pairs or elbow buddies, for 2 minutes, what they observed during the teacher demo and what they think was happening.
Day 2 (40 minutes): Explore outside, collect leaves, and set up scientific journal
- As a class, pose the question: why it is important to do chromatography on leaves. Have students brainstorm their ideas or talk with a peer before listening to their responses. Tell students that chromatography allows us to see the different colors that make up a leaf.
- Before going outside,
- give students a bag to collect leaves and add any they collected from home.
- tell students that they will use their notebooks to record their observations.
- have students set up graphs in their science notebooks.
- Have students either cut and glue the Chromatography #1 Worksheet provided into their notebooks or have them draw the graph into their notebooks).
- Ask students to write down a description of a leaf
- Have students draw the leaf in their science notebook.
- Instruct students that they need to collect 3-5 leaves of the same tree/type.
- Review the Outdoor Investigation Rubric with the students before going outside.
- Take students outdoors and conduct their outdoor exploration to gather their leaves.
- Return to the classroom after students find their leaves.
- In the classroom, have students write down information about their collected leaves on their Outdoor Investigation Sheet (or, in an optional notebook).
- In small groups, give students time to discuss their leaf findings. Each group then votes for two leaves for chromatography tests in the next activity.
Day 3 (40 minutes): Running Chromatography Day 1
- Tell students they will be using the chromatography technique that they saw with the purple skittle teacher demonstration, but it will be done slightly differently.
- Discuss how chromatography allows us to separate a mixture.
- Ask: Who remembers what a mixture is? (Allow for students to respond). Explain that a mixture is when two or more substances are combined. When we use chromatography we have the stationary phase (chromatography paper) and a mobile phase (isopropyl alcohol) that helps move the different components of the mixture up the paper. The different chemical compounds will travel up the paper at different rates depending on their solubility. If a substance (A) is very soluble (meaning that it can be dissolved in a solvent more than other compounds) it will travel farther up the paper and if a compound (B) is less soluble (meaning that it is harder to be dissolved in a solvent) it won’t travel as far up the paper.
- Tell the students they are going to be investigating the different pigments in the leaves they have collected from yesterday.
- Have students set up their science notebook. (Students can either cut and glue the Chromatography #1 Worksheet provided into their notebooks or draw the table into their notebooks). Tell students to be sure to tape or draw one of the leaves from the two kinds of leaves their group voted on into the first box. Have students write down the number of leaves they will use in their mixture, the amount of solvent in mL they will be using (between 6-10 mL of isopropanol) in their test, and their results/what they observed after running the chromatography.
- Hand out materials to each group.
- Tell students that they will be running two different chromatography trials for two different types of leaves. Give students the option of having their 4-person group split off into 2 students running chromatography on one type of leaf and the other 2 students running chromatography on the second type of leaf.
Steps students should take to conduct their investigation:
- After putting on safety goggles, grab the first set of leaves (3-5 leaves) and cut and crush them with their hands and then place them in the mortar.
- Measure 6 mL of isopropyl alcohol in the graduated cylinder and then pour into the mortar.
- Using the pestle, grind the leaves until the pigment of the leaves begins to intensify.
- Using a ruler, students cut out 1 strip of chromatography paper (or teacher can cut these beforehand.)
- In pencil, write the date on the top of the strip, the leaf name and/or at least a distinguishing factor (ex: leaf #1, leaf #2, red leaf, green leaf, etc.).
- Transfer the mixture of leaves and solvent into the glass jar leaving the big chunks of leaves behind in the mortar.
- Gently place the chromatography paper into the glass jar and secure it by using the mini binder (or mini clothespin) and clipping the paper to the top of the jar. This will reinforce the paper to remain straight and not slump or bend in the jar.
- Cover the top of the jar with Saran wrap
- Observe and take notes in your science notebook making sure to watch the color pigments and the solvent moving up the chromatography paper.
- Once the pigments have traveled far enough up the paper that you can identify the different colors, take the chromatography paper out of the jar, and place it on the table to dry.
- Write down any last notes and observations.

- Have students share their information and present their findings to their group members. Have students complete the Exit Ticket provided.
Day 4 (40 minutes): Running Chromatography Day 2
- Read aloud a few of the exit tickets and discuss the information one can gather when running chromatography on leaves.
- Explain that plants use light energy from the sun to produce the food they need to survive. This process is called photosynthesis. The plant’s cells then absorb the light from the sun. Inside some of these cells are compounds called chlorophyll. Chlorophyll absorbs the sunlight to start photosynthesis. Often when one looks at plants and leaves, they tend to mostly be green.
- Discuss what happens to the color of the leaves in the fall. Have students brainstorm or talk with a peer before listening to their responses. Explain that leaves begin to change color into orange, yellow, and even red. Discuss why this might happen. Have students brainstorm or talk with a peer before listening to their inferences. Explain that it happens because other pigments that have been there started to appear while the chlorophyll breaks down. Carotenoids is the compound that creates the orange pigment in leaves. Xanthophylls is a type of carotenoids, it has a compound called lutein, that creates the yellow pigment in leaves. There is also red pigment in the leaves that comes from the compound anthocyanin. Ask: what can one do to be able to see these colors in a green leaf that will change color in the fall? Explain that one can do chromatography to see these hidden colors!
- Tell students that today they will do chromatography on a few different leaves that the teacher collected for them. Explain that each group will have different leaves and they will be running chromatography on these new leaves just like they did previously on the ones they found outside. Have students set up their science notebook. (Students can either cut and glue the Chromatography #2 Worksheet provided into their notebooks or draw the table into their notebooks).
- Students will repeat steps from yesterday with the new leaves to conduct their investigation.
- Have students share their information and present their findings to their group members.


Day 5 (40 min): Create Reflection Project (Post-assessment)
- Have students complete the Check-in Ticket provided.
- Review what photosynthesis is, chlorophyll, and explain that we can use chromatography to see the other pigments in leaves.
- Tell students that with their investigation and research being done they will now be creating a project to showcase what they learned, the process they took, and their findings through their investigation and experimentation.
- Review the Reflection Project Rubric with students.
- Instruct students to write their findings down in their science journals.
- Have students create a presentation using one of the following: Google Slides, PowerPoint, a video, pamphlet, or a poster which they will then use to showcase their results. Explain that they will need approval from the teacher on which medium they will be using before they begin working on their presentation.
- Students can begin to work on their findings and reflection project.
Day 6 (40 min): Continue working on Reflection Project
- Tell students that they will have this class period to continue working on their post-assessment and to put their finishing touches on their projects before presenting them tomorrow.
Day 7 (40 min): Presentation of Learning/Results and Reflection Project
- Tell students that today they will be presenting their findings and results in the form of a gallery walk.
Vocabulary/Definitions
chlorophyll: Compound found in cells of plants that absorb sunlight to begin photosynthesis.
chromatography: A process in which a dissolved mixture of various chemical compounds passes through a form, such as paper, separating the chemical compounds based on the different rates they move.
photosynthesis: Process in which a plant uses light energy from the sun to produce food they need to survive.
pigment: The natural color of matter in plant tissue or animals.
solute: A substance, chemical compound that is dissolved in a solution.
solution: A mixture made of two or more substances.
solvents: The major component of a solution and it will dissolve a solute.
Assessment
Pre-Activity Assessment
Day 1 Pre-Assessment: Students will answer a few questions about mixtures, solutions, and plants in the Pre-Assessment.
Activity Embedded Assessment
Day 3 Exit Ticket: Students answer a quick question about running chromatography in the Exit Ticket.
Day 5 Check-in Ticket: Students answer two questions about chlorophyll and photosynthesis in the Check-in Ticket.
Post-Activity Assessment
Reflection Project: Students will be creating a project to showcase what they learned, the process they took, and their findings through their investigation and experimentation.
Safety Issues
Outdoor exploration - make sure that there aren’t any thorny, spikey, or poisonous leaves in the exploration area.
Isopropyl Alcohol - make sure students avoid contact with eyes and be sure to properly dispose of the isopropyl alcohol as flammable waste.
Troubleshooting Tips
Students will most likely collect more than two types of leaves—this is fine. They should have enough space in their journal to document the amount of leaves they collected in their science journals. If students collect a lot more than the amount in rubric (3-5 leaves), it is okay.
Some recommended leaves that will show a different color on the chromatography paper include:
- Ginkgo (when it is green) will show yellow on paper.
- Tradescantia pallida, “Purple Heart” (when it is purple) will show some green and brown after running chromatography.
- Heavenly Bamboo Gulf Stream (when it is mostly red) will show some yellow
It may be best to have the students write observations the following day after running chromatography on leaves once the chromatography paper has dried.
Teacher can decide if they want students to repeat the chromatography process (on day 4) with leaves they collected or have students continue to do chromatography with leaves that the students have collected (if they collected more than just 2 types of leaves).
Activity Scaling
- For lower grades, students can use markers or different types of fruit or food to learn about pigments.
- For higher grades, students can further explore the reason behind why chromatography would be useful (such as pharmaceutical industries, food industry, forensics investigations, etc.)
Additional Multimedia Support
Video on Explanation of the Basics of Paper Chromatography and Thin Layer Chromatography (https://vimeo.com/182048032)
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn how to classify materials as mixtures, elements or compounds and identify the properties of each type. The concept of separation of mixtures is also introduced since nearly every element or compound is found naturally in an impure state such as a mixture of two or more substances, and...

To increase students' awareness of possible invisible pollutants in drinking water sources, students perform an exciting lab requiring them to think about how solutions and mixtures exist even in unsuspecting places such as ink. They use alcohol and chromatography paper to separate the components of...
References
Benson, A. A. Bassham, J. A. Calvin, M. Goodale, T. C. Haas, V. A. and Stepka W. “The Path of Carbon in Photosynthesis. V. Paper Chromatography and Radioautography of the Products.” Journal of the American Chemical Society 1950 72 (4), 1710-1718
DOI: 10.1021/ja01160a080
Brunning, Andy. “The Chemistry of Autumn Leaf Colours.” Compound Interest, Compound Interest 2018, 11 Sept. 2014, www.compoundchem.com/2014/09/11/autumnleaves/.
Buddies, Science. “Find the Hidden Colors of Autumn Leaves.” Scientific American, 20 Oct. 2011, www.scientificamerican.com/article/bring-science-home-leaf-colors/.
Education.com. “Candy Chromatography: Science Project.” Science Project | Education.com, 6 Jan. 2014, www.education.com/science-fair/article/chemistry_paper-chromatography/?source=related_materials&order=1.
Education.com. “Leaf Chromatography: Science Project.” Science Project | Education.com, 29 Jan. 2014, www.education.com/science-fair/article/find-color-pigments-hidden-green/.
“Leaf Chromatography Science Experiment for Fall STEM Activities.” Little Bins for Little Hands, 11 Sept. 2017, littlebinsforlittlehands.com/leaf-chromatography-science-experiment-stem-activities/.
Palm , C. E. (n.d.). Around Your World. Retrieved from https://www.esf.edu/pubprog/brochure/leaves/leaves.htm
Copyright
© 2020 by Regents of the University of Colorado; original © 2019 University of Texas at AustinContributors
Melissa Saldaña; Stephanie ValenzuelaSupporting Program
The Research Experience for Teachers program hosted by the Center for Dynamics and Control of Materials and NSF Materials Research Science and Engineering Center, University of Texas at AustinAcknowledgements
This work is based upon work supported primarily by the National Science Foundation under Cooperative Agreement No. EEC-1160494. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. Support for this research was provided by the National Science Foundation through the Center for Dynamics and Control of Materials: an NSF MRSEC under Cooperative Agreement No. DMR-1720595.
Last modified: June 13, 2025




User Comments & Tips