Quick Look
Grade Level: 11 (9-12)
Time Required: 45 minutes
Lesson Dependency: None
Subject Areas: Earth and Space, Science and Technology
NGSS Performance Expectations:

| HS-ESS3-1 |

Summary
This lesson conveys core information about why air quality is important and how engineers tackle complex environmental problems—providing a foundation for the subsequent five activities. Students learn the basics about the structure of the Earth’s atmosphere, the types of pollutants that are present in the atmosphere (primary, secondary, gas-phase compounds, particulate matter), and the importance of air quality research. They are also introduced to some engineering concepts such as how air quality measurements are made and how control technologies work. A PowerPoint® presentation, teacher slide notes, blank vocabulary list, post-lecture quiz, homework handout, and a pre-unit STEM survey are provided. This lesson and its five associated activities are intended to prepare and guide students to take on their own research projects.Engineering Connection
Engineers are problem solvers. Poor air quality poses a problem for human and environmental health and finding good solutions requires a thorough understanding of the problem at hand. During this lesson, students learn what air pollutants are, as well as the scientific principles and technologies that enable engineers to measure and mitigate these pollutants. The background information provides the foundation from which environmental engineers work: pollution types, pollution sources, measurement technologies, and control strategies. Students are also introduced to the technology used in the subsequent activities—a low-cost air quality monitor that was developed and is used by mechanical and environmental engineers at the University of Colorado Boulder.
Learning Objectives
After this lesson, students should be able to:
- List and describe the three primary reasons to study air quality: health effects, aesthetics and climate change.
- Explain the differences between primary and secondary pollutants, and gas-phase and particulate pollutants.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ESS3-1. Construct an explanation based on evidence for how the availability of natural resources, occurrence of natural hazards, and changes in climate have influenced human activity. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Construct an explanation based on valid and reliable evidence obtained from a variety of sources (including students' own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future. Alignment agreement: | Resource availability has guided the development of human society. Alignment agreement: Natural hazards and other geologic events have shaped the course of human history; [they] have significantly altered the sizes of human populations and have driven human migrations.Alignment agreement: | Empirical evidence is required to differentiate between cause and correlation and make claims about specific causes and effects. Alignment agreement: Modern civilization depends on major technological systems.Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the effects of technology on the environment.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Assess how similarities and differences among scientific, mathematical, engineering, and technological knowledge and skills contributed to the design of a product or system.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Assess a technology that minimizes resource use and resulting waste to achieve a goal.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Develop a solution to a technological problem that has the least negative environmental and social impact.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
Colorado - Science
-
The interaction of Earth's surface with water, air, gravity, and biological activity causes physical and chemical changes
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Climate is the result of energy transfer among interactions of the atmosphere, hydrosphere, geosphere, and biosphere
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/cub_airquality_lesson01] to print or download.Introduction/Motivation
(Be ready to present to the class the 32-slide Air Quality Introduction Presentation, a PowerPoint® file, after presentation of the Introduction/Motivation content. The slides are “animated,” so clicking the mouse or space bar brings up the next item. Refer to the slide notes below each slide for lecture content; these notes are also provided in a separate document, Introduction Presentation Slide Notes, in case you want to print them out. Also make copies of the Post-Lecture Quiz and direct students to fill it out either during the slide presentation or immediately following. After the lecture and assessment, proceed to conduct the associated activities.)
How many of you have ever been stuck behind an old semi-truck on the road and seen black smoke billowing out of its tail pipe? (Wait for a show of hands. Listen to any student comments.) Now, what do you think is in that smoke? What is it composed of? (Ask for guesses and start making a list on the classroom board; below are the responses you are looking for and elaborations on each.)
(First, share an explanation of what happens when you burn fuel.) Burning fuel results in a chemical reaction and a change in the composition of the fuel. Fuels are primarily made of carbon and hydrogen and whenever you burn them, water vapor and various pollutants are produced.
So what might we find in vehicle exhaust fumes?
- Water vapor (H2O) is always produced during combustion. (Refer to the associated activity Combustion and Air Quality: Emissions Monitoring for students to gain understanding in different types of vehicle emissions). You might find it interesting to learn that modern power plants typically have so many control technologies in place that most of what is emitted via the smoke stacks is water vapor! (Note: Control technologies remove pollutants from the waste emissions before they leave the plant; slides 27-32 provide specific examples.)
- Particulate matter (PM) is what makes smoke visible. These are tiny pieces of fuel that have been broken up by the combustion process but not completely burned.
- The final product of ideal or complete combustion is carbon dioxide (CO2). This pollutant is a major greenhouse gas and thus a concern for climate change.
- Carbon monoxide (CO) is another pollutant that is produced when a fuel is not completely burned up, like PM. This gas is a major danger to human health. You might have carbon monoxide detectors in your homes for this reason.
The very common example of smoke from a tailpipe illustrates how air quality intersects your everyday life. Today, we will cover an introduction to air quality research, measurements, and control technologies to provide you with a foundation from which to undertake your own air quality projects later in the term.
(Next, present to the class the Air Quality Introduction Presentation.)
Lesson Background and Concepts for Teachers
The following information provides an introduction to the concepts covered in the Air Quality Introduction Presentation. In addition, lecture notes are provided with each slide, as well as in a separate file, Introduction Presentation Slide Notes, which you may want to print out.
The Basics
Without our atmosphere, life on Earth would not exist. Our atmosphere is a complex system that interacts with the hydrosphere, geosphere and the biosphere. For example pollutants can move from the hydrosphere and into the atmosphere; from there they can be transported and impact the biosphere. None of these systems are isolated.
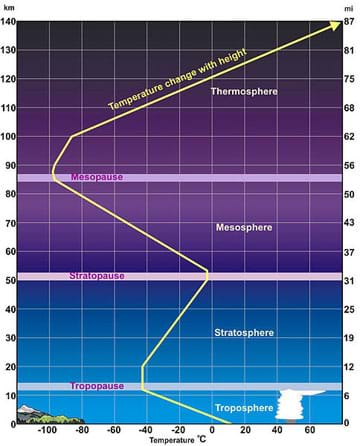
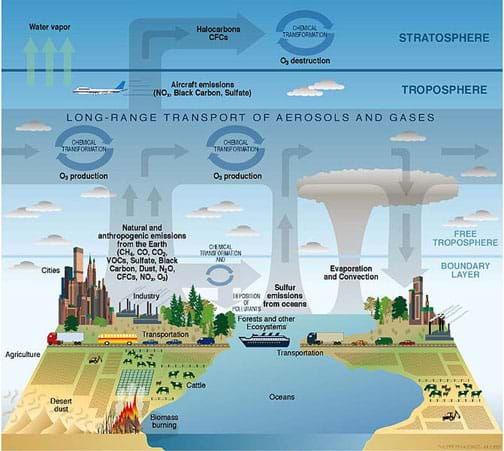
In terms of structure, the atmosphere is composed of layers that are defined by the temperature profile in relation to elevation (as shown in Figure 1). In Figure 1, the yellow line shows the changing temperature at different elevations in the various layers. Complex atmospheric dynamics determine how air moves within and between these layers. It is important for researchers to understand the atmosphere in order to better predict the fate and transport of airborne contaminants (see Figure 2).
Air Quality
Our atmosphere is ~21% oxygen and ~78% nitrogen; the remaining 1% is considered “trace gases” and this includes everything else—from carbon dioxide to the noble gases like argon. Scientists and engineers study this 1%, as well as the many types of particles present in the atmosphere. When we think of air quality, we typically think of the air we are breathing and whether or not it is safe. However, air quality can refer to ambient outdoor conditions, indoor conditions, particular sources, good air quality vs. poor air quality, etc. Within the field of air quality, researchers specialized in many other specific topics. Due to the complexity of our atmosphere and the possibility of transport over long distances, we can also think of air quality in terms of scale—that is, pollution may cause local or global problems. For example, pollution in China can make its way over North America and add to existing pollutants there.
Poor air quality can negatively affect human and environmental health. In humans, poor air quality can lead to a multitude of problems that include respiratory and cardiovascular diseases. We tend to think first of asthma and respiratory problems, but some particles are so small that they can enter the blood stream through the lungs and cause inflammation leading to issues beyond our breathing. In plants, poor air quality can also cause disease that can result in crop loss. In addition to human and environmental health, many pollutants that we worry about are greenhouse gases and contribute to climate change. Finally, poor air quality can impact quality of life. Consider visibility issues in National Parks and odors near industrial areas of cities; in addition to potential health dangers, these air quality issues can make daily life unpleasant.
Pollutant Types
We tend to think of pollutants as either particles (that is, particulate matter, like dust) or gas-phase compounds. Gas-phase compounds are molecules composed of multiple atoms that exist in the gas phase (carbon dioxide is an example of a gas-phase pollutant). Particulate matter varies in size and composition. Very small particulate matter is an accumulation of either solid or liquid molecules. Examples of larger particulate matter are dust and pollen. Refer to the associated activity Linking Sources and Pollutants to give students a basic understanding of how everyday products such as rubbing alcohol and burning wood emit various pollutants.
Another important distinction in air pollutants is between primary and secondary emissions. Primary emissions are direct emissions. For example, the pollutants from a tailpipe or smokestack are primary emissions, whereas secondary pollutants form in the atmosphere. A prime example is ground-level or tropospheric ozone, which requires several primary pollutants and sunlight to be created. Atmospheric chemistry is also complex, and what we emit may disperse, may react and become something entirely different, or may stick around for a long time, depending on the particular compound and environmental conditions.
Air Quality Measurements
Engineers and scientists take advantage of the properties of different pollutants to quantify the problems those pollutants cause. Particulates can be captured on a filter, which can then be weighed or analyzed. We can also take advantage of how different sized particles behave to separate them. Gases also have properties we can take advantage of for measurement purposes, such as absorbance, for example. Some gases absorb particular wavelengths of light, and by measuring what is absorbed by a sample, we can determine the amount of gas present. The ability to measure air pollutants is necessary first to set regulations and then to ensure the regulations are enforced for the protection of human and environmental health.
Next-generation air quality monitoring technologies are the primary tool used throughout this unit. Advances in sensor technology have made low-cost equipment possible. While these technologies are not as reliable as higher-cost conventional monitoring technologies, they make the collection of data with higher spatial and temporal resolution possible. Additionally, lower-cost technologies make monitoring more accessible to developing countries, communities, schools and citizen scientists. Students can further investigate the importance and processes of monitoring air quality with the associated activity Understanding the Air through Data Analysis. Follow with the associated activity Study Design for Air Quality Research where using a case study, students practice planning a project that compares traditional cook stoves to new and improved cook stoves for use in the developing world. Students can then use the associated activity Communicating Your Project Results with Professional Posters to help convey their final findings.
Air Pollution Control Technologies
Engineering really comes into play in the role of designing and implementing air pollution control technologies. For example, technologies like catalytic converters in cars make it possible for us to continue using fossil fuels, manufacturing, and building, while minimizing the harmful emissions. This approach reduces the CO and VOCs emitted through oxidation reactions; by oxidizing these components, more of the emissions are converted to CO2. Other technologies: Particulate matter (PM) can be captured in filters, gases can be combusted to remove products of incomplete combustion, emissions can even be cleaned by flowing dirty air through chambers spraying water (scrubbers). The major improvements to U.S. air quality since the 1960s are largely due to regulations such as the Clean Air Act, and subsequent improvements/applications of control technologies.
Appendix Slides
At the end of the Air Quality Introduction Presentation there is additional information specific to the Pod air quality monitor. We recommend the teacher review it in advance and then share with students any information deemed useful. The slides include instructions for using the Pod, an explanation of how the sensors work, information on calibration, and a discussion of example data.
Associated Activities
- Linking Sources and Pollutants - Students acquire a basic understanding of how different everyday items such as rubbing alcohol and burning wood emit various pollutants. This helps them to connect the pollutants they learned about in the lesson with potential real-world sources. Teams make predictions, collect data, and evaluate the results. Students gain familiarity with the use of a low-cost air quality monitor (rentable Pods).
- Combustion and Air Quality: Emissions Monitoring - Students use low-cost air quality monitors (Pods) to measure the emissions from different vehicles. By applying the knowledge about combustion chemistry they gained during the pre-activity work, students predict how the vehicle emissions for different pollutants will differ and explain why. After data collection, they examine time series plots of the data and discuss the results as a class, covering results interpretation and comparison of results to predictions.
- Study Design for Air Quality Research - Students take an in-depth look at what goes into planning research projects in order to prepare them to take the lead on their own projects. Using a case study, students practice planning a project that compares traditional cook stoves to new and improved cook stoves for use in the developing world. Then they compare their plans to one used in the real-world by professional researchers. Then groups are provided with materials and support to take them from brainstorming to completing detailed research plans for their own air quality research projects (may take days, weeks or months), which teams conduct after this activity and before the final activity in the unit.
- Understanding the Air through Data Analysis - Students apply their existing air quality knowledge and a description of a data set (measuring carbon dioxide or ozone) to each develop a hypothesis around how and why air pollutants vary daily and seasonally. A worksheet-guided Excel-based analysis of the data includes entering formulas to calculate statistics and create data plots. At each analysis phase, reflection questions prompt students to new information the analysis reveals. At activity end, students evaluate their original hypotheses and “put all of the pieces together.”
- Communicating Your Project Results with Professional Posters - To conclude the unit, students create scientific research posters presenting the results of their AQ-IQ research projects. First they critically examine example posters to gain an understanding of what to include and how they can be made most effective. Then they analyze and interpret their data, including what statistics and plots to include in the posters. Finally, teams are given a poster guide that prompts them about all of the elements one would find in any research paper or professional presentation. Then groups present their research study results in poster format to their peers or a wider audience.
Vocabulary/Definitions
carbon dioxide: A colorless and odorless gas. A gas-phase pollutant. Composed of 1 carbon atom and 2 oxygen atoms. Generated by the respiration of animals and the combustion (burning) of fuels that contain carbon. Abbreviated as CO2.
carbon monoxide: A colorless, odorless and tasteless gas. A compound that is a product of incomplete combustion and is dangerous to human health. Composed of 1 carbon atom and 1 oxygen atom. Abbreviated as CO.
control technologies: Technologies that capture or change pollutants in emissions from cars, factories, power plants, and oil and gas operations, thereby resulting in cleaner final emissions. Example: catalytic converters in cars.
gas-phase pollutant: A compound comprised of multiple atoms (such as carbon dioxide with 1 carbon and 2 oxygens) existing in the gaseous physical phase.
greenhouse gas: A gas in the atmosphere that absorbs certain wavelengths of light, thereby radiating that heat back into the atmosphere, as opposed to not absorbing the light so that it escapes the atmosphere.
hydrocarbon: A compound that contains only carbon and hydrogen atoms. Another term for VOC. Abbreviated as HC.
mitigation: In terms of pollution, limiting the amount of a pollutant emitted or produced; this may be done through better technologies, regulation changes or attempts to change human behavior.
monitoring technology: In terms of pollution, technology and tools that engineers and scientists use to quantify exactly how much of a particular pollutant exists either indoors or outdoors.
nitrogen dioxide : A gas-phase compound made of 1 nitrogen atom and 2 oxygen atoms. It is formed during high-temperature combustion from the nitrogen that exists in the air. High-temperature combustion also produces nitrogen monoxide (NO). The sum of the amount of NO and NO2 is the amount of NOx present; in other words NOx is a term that includes both NO and NO2.
organic compound: In chemistry, any compound that contains carbon atoms. For example, living things are organic, while rocks are inorganic.
ozone: A pale blue gas with a distinctively pungent smell. It is a secondary pollutant formed by NOx and VOCs in the presence of sunlight. Dangerous to human health at ground level, but high in the stratosphere it protects humans from harmful UV rays. Mnemonic: “good up high, bad nearby.”
particulate matter: A microscopic solid or liquid compound that may be natural or human-made. Very small particulate matter may be a conglomerate of gas-phase compounds; larger particulate matter can be dust or pollen.
primary pollutant: A pollutant that is directly emitted by a source. For example, exhaust from a tailpipe or smokestack, material erupted from a volcano, or the CO2 exhaled by humans.
secondary pollutant: A pollutant that is formed via atmospheric chemistry from the byproducts of primary emissions.
temperature inversion: When a warm air mass moves on top of a cold air mass, creating stagnate conditions, which can prevent dispersion and trap pollutants.
volatile organic compound: An organic chemical that has a high vapor pressure at ordinary room temperature, such that it volatizes (enters the gas phase) at room temperature and pressure. An example is formaldehyde (CH2O, 1 carbon, 2 hydrogens, and 1 oxygen atom). Abbreviated as VOC. VOCs are also gas-phase compounds. VOCs also include products of incomplete combustion (when a carbon-fuel is not completely burned, resulting in only CO2).
Assessment
Pre-Lesson Assessment
Vocabulary List: Have students look over the Vocabulary List and complete as many terms as possible. Before beginning the presentation, call on students to guess what the various terms mean. Then throughout and after the slide presentation lecture, have students fill in the missing definitions and correct any misunderstood terms. Suggest that students use this list as a valuable reference through all subsequent activities.
Post-Introduction Assessment
Post-Lecture Quiz: Immediately after the slide presentation lecture, administer the five-question Post-Lecture Quiz, which ties directly to the learning objectives and can be completed either individually or in pairs. Then either have students turn in the quizzes for grading or discuss the answers as a class.
Pre-Unit STEM Survey: After students complete this lesson and its first associated activity, administer the six-question STEM Survey 1. Then, after students complete the last (fifth) associated activity, Communicating Your Project Results with Professional Posters, administer its STEM Survey 2. Most of the questions are the same, which enables a pre/post comparison of the impact of the unit and project on students’ attitudes and knowledge of STEM and engineering.
Lesson Summary Assessment
Vocabulary List: Grade students’ completed Vocabulary List for credit; or have students submit a pre-version and a post-lecture version of the list (all of the terms are covered in the presentation). Alternatively, expand the assessment by making, separating and shuffling terms and definitions into a matching assessment.
Explanation: Have students construct an explanation based on what they learned for how the availability of natural resources and changes in climate have influenced human activity.
Homework
Connecting to the Big Picture: At the end of class, have students complete the three-question Connecting to the Big Picture Handout as homework. If possible, take 10 minutes at the beginning of the next class period to discuss their answers. The questions are open-ended and intended to prompt students to reflect on connections between what they are learning and everyday life. If your students are completing the entire AQ-IQ curriculum, these questions are recommended to help them begin to think about topics they may wish to focus on for their projects. As an alternative, save this assessment to assign to students as part of the next associated activity, Linking Sources and Pollutants.
Additional Multimedia Support
Air Quality & Pollutants
The U.S. Environmental Protection Agency has great online resources covering air quality issues (indoor and outdoor), pollutants (what they are, sources and regulations), as well as, teacher resources.
- The basics of AQ: https://www.epa.gov/environmental-topics/air-topics
- Links to information on specific pollutants: https://www.epa.gov/environmental-topics/air-topics
- The home page for indoor air quality information: https://www.epa.gov/indoor-air-quality-iaq
- Teacher resources developed by the EPA: https://www.epa.gov/new-bedford-harbor/environmental-education-resources-teachers-and-students
- Air Quality Data by Country: https://openaq.org/#/countries?_k=591ioq
Resources on the Atmosphere and Climate
Below are resources developed by the University Corporation for Atmospheric Research (UCAR) and the University of Colorado Boulder.
- Homepage for UCARs “Learning Zone” resources: https://scied.ucar.edu/resources
- Background information on the atmosphere: https://scied.ucar.edu/learning-zone/atmosphere
- Background information on the Earth’s climate: https://scied.ucar.edu/learning-zone/how-climate-works/climate
Air Quality Data
- AirNow: U.S. air quality data provided in concentrations and visualized on maps with information on health risks associated with certain levels: https://www.airnow.gov/
- Home page for the CDPHE’s (Colorado Dept. of Public Health and the Environment) resources on Colorado air quality, including live data streams: https://www.colorado.gov/airquality/
- Live map of CDPHE air quality data: http://www.colorado.gov/airquality/all_sites_map_ags.aspx
Sites Run by the Hannigan Research Lab at CU-Boulder
- The wiki site for education and outreach activities: https://www.colorado.edu/aqiq/
- The lab’s research group page: https://www.colorado.edu/lab/hannigan/
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are introduced to the concepts of air pollution, air quality, and climate change. The three lesson parts (including the associated activities) focus on the prerequisites for understanding air pollution. First, students use M&M® candies to create pie graphs that express their understanding o...

Students are presented with examples of the types of problems that environmental engineers solve, specifically focusing on air and land quality issues.

Looking at transportation and the environment, students learn that some human-made creations, such as vehicles, can harm the natural environment. They also learn about alternative fuels and vehicles designed by engineers to minimize pollution. The associated hands-on activity gives students a chance...

As a class, students use a low-cost air quality monitor (a rentable “Pod”) to measure the emissions from different vehicles. By applying the knowledge about combustion chemistry that they gain during the pre-activity reading (or lecture presentation, alternatively), students predict how the emission...
References
Collier, A. M., D. W. Knight, K. Hafich, M. P. Hannigan, B. Graves and M. Polmear, “The North Fork Valley Project: A Project-Based Learning Curriculum to Support the Use of Next-Generation Monitoring Technologies in Rural Communities,” American Society for Engineering Education, Rocky Mountain Section Conference, Denver, CO, April 2015.
Collier, A. M., D. W. Knight, K. Hafich, M. P. Hannigan, B. Graves and M. Polmear, “On the Development and Implementation of a Project-Based Learning Curriculum for Air Quality in K-12 Schools,” Frontiers in Education Conference, El Paso, TX, October 2015.
Other Related Information
AQ-IQ Air Quality Monitor Information
This curriculum was designed to support high school students’ use of a low-cost air quality monitor developed by the Hannigan Lab at the University of Colorado Boulder called a “Pod.” Pods can be rented and shipped from the university; see below for details. Alternatively, many of the activities, including the long-term project, can be completed with other air quality monitors—or no monitor. For example, students can design research projects that utilize existing air quality data instead of collecting their own, which is highly feasible since a great amount of data from around the planet is publically available. In addition, other low-cost monitors could be used instead of the Pods, ranging from purchasable to DIY; see below for a list of options.
Is an air quality monitor needed for this lesson and its first associated activity?
No monitors are required for this lesson, which covers foundational air quality background information. However the next associated activity, Linking Sources and Pollutants, was designed to be completed with a monitor. If you are not able to obtain an air quality monitor at this point in time, but would like to introduce the material, another good alternative activity to accompany this lesson is an AP Environmental Science Air Pollution Lab activity in which students combust a range of household materials to generate small quantities of various primary air pollutants and make observations of the by-products, such as smoke, odor, ash and sound. This exercise prompts students to think about the breadth of air pollutants in our atmosphere by making pollutants visually observable in a lab setting.
Copyright
© 2013 by Regents of the University of ColoradoContributors
Ashley Collier; Katya Hafich; Daniel Knight; Michael Hannigan; Joanna Gordon; Ben Graves; Eric Ambos; Olivia Cecil; Victoria Danner; Erik Hotaling; Eric Lee; Drew Meyers; Hanadi Adel Salamah; Nicholas VanderKolkSupporting Program
AirWaterGas SNR Project Education and Outreach, College of Engineering, University of Colorado BoulderAcknowledgements
This material is based upon work by the AirWaterGas Sustainability Research Network Education and Outreach Project in the College of Engineering at the University of Colorado Boulder, supported by National Science Foundation grant no. CBET 1240584. However, these contents do not necessarily represent the policies of the National Science Foundation, and you should not assume endorsement by the federal government.
The authors also express their appreciation for the support of the University of Colorado’s Office of Outreach and Engagement.
Last modified: July 17, 2023






User Comments & Tips