Quick Look
Grade Level: 12 (10-12)
Time Required: 45 minutes
(Plus a brief follow-up the next day to record siphon results and wrap up the worksheet.)
Expendable Cost/Group: US $8.50 This activity requires some non-expendable items typically available in high school chemistry lab classrooms; see the Materials Lists for details.
Group Size: 3
Activity Dependency: None
Subject Areas: Chemistry, Physical Science, Physics
NGSS Performance Expectations:

| HS-ETS1-3 |
| HS-PS1-3 |
Summary
Students observe multiple examples of capillary action. First they observe the shape of a glass-water meniscus and explain its shape in terms of the adhesive attraction of the water to the glass. Then they study capillary tubes and observe water climbing due to capillary action in the glass tubes. Finally, students experience a real-world application of capillary action by designing and using "capillary siphons" to filter water.Engineering Connection
Capillary action in, part, determines the behavior of ground water in soil, which makes understanding the concept important to both civil and environmental engineers in their work to ensure the stability of buildings and roads as well as understand the environmental impact of human development. Petroleum engineers use their understanding of capillary action in the extraction of crude oil from rock reservoirs. The behavior of capillary action is exploited in blood sugar test strips to help diabetics monitor their conditions.
Learning Objectives
After this activity, students should be able to:
- Explain what a meniscus is and how it is formed.
- Describe how the combination of adhesive and cohesive forces causes water to rise in a thin tube or other confined space (capillary action).
- Design a "capillary siphon" that filters water by using capillary action to transfer water from a "cloudy" to a "clean" bucket.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-3. Evaluate a solution to a complex real-world problem based on prioritized criteria and trade-offs that account for a range of constraints, including cost, safety, reliability, and aesthetics, as well as possible social, cultural, and environmental impacts. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Evaluate a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | When evaluating solutions it is important to take into account a range of constraints including cost, safety, reliability and aesthetics and to consider social, cultural and environmental impacts. Alignment agreement: | Models (e.g., physical, mathematical, computer models) can be used to simulate systems and interactions—including energy, matter, and information flows—within and between systems at different scales. Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS1-3. Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly. Alignment agreement: | The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms. Alignment agreement: | The functions and properties of natural and designed objects and systems can be inferred from their overall structure, the way their components are shaped and used, and the molecular substructures of its various materials. Alignment agreement: |
Common Core State Standards - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Summarize, represent, and interpret data on a single count or measurement variable
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Evaluate ways that technology can impact individuals, society, and the environment.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
State Standards
North Carolina - Math
-
Reason abstractly and quantitatively.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Summarize, represent, and interpret data on a single count or measurement variable
(Grades
9 -
12)
More Details
Do you agree with this alignment?
North Carolina - Science
-
Explain the effects of forces (including weight, normal, tension and friction) on objects.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Compare physical and chemical properties of various types of matter.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
For the introductory teacher demonstration:
- 2 large beakers
- reptile substrate or bedding from a pet store, enough for the two beakers (this material comes in desiccated [dried out] bricks of plant matter; the night before, add water to one beaker to thoroughly wet the material overnight)
- water
- cup, to hold and pour water into the beakers during the demo
Each student needs:
- Capillary Action Activities Worksheet
- eye protection glasses or goggles
For Part I: The Meniscus, each group needs:
- graduated cylinder
For Part II: Capillary Tubes, each group needs:
- ring stand with support ring attached
- 3 capillary tubes of different diameters
- large Petri dish
- twist-ties
- water with dark food coloring
- ruler
For Part III: Using Capillary Action, each group needs:
- assortment of various types of paper, cloth and string
- paper towels
- scissors
- tape
- 2 buckets
- ruler
- scale
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/duk_surfacetensionunit_act2a] to print or download.Pre-Req Knowledge
A basic understanding of adhesive and cohesive forces, surface tension and capillary action.
Introduction/Motivation
(The night before, fill two large beakers with the dry [desiccated] reptile substrate or pet bedding material. Add water to one beaker, so it is no longer dry by the morning.)
(Conduct the pre-activity assessment with water droplets and paper towels, as described in the Assessment section.)
(Hold up and show students the beaker with the dry material in it.) What do you think will happen if I add a cup of water to this? (Expect students to predict that it turns into mud. Pour in the cup of water. Instead, when you first pour it in, it runs straight through the "dirt" into the bottom of the beaker.)
Now, if I left this overnight, what would happen? (Show students the other beaker, with the material soaked in water overnight.)
What has happened? Why has this dirt become moist? (Listen to student ideas.) Given time, the water has moved into all of the crevices because of its attraction to the material. This is another example of capillary action. This is also a quick demonstration of the movement of groundwater in soil.
Today, we will explore different aspects of capillary motion and how water can creep from one place to another.
Procedure
Background
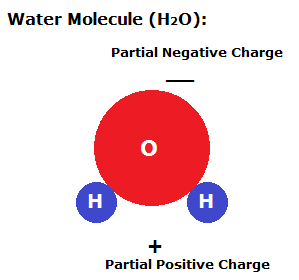
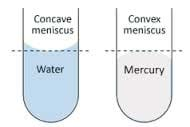
Consider a glass tube placed in a cup of water. Inside the tube, adhesive forces draw the water up the sides of the glass tube to form a meniscus. Cohesive forces then try to minimize the surface area by drawing water up the tube against gravity. This rise in the water level in a tube due to adhesive and cohesive forces is known as capillary action.
Water moves through any confined space by capillary action. When you place a paper towel on a water spill, the water is drawn into the paper as it moves into the small gaps between paper fibers. These small gaps give cloth, sponges and paper towels their absorbency. The capillary action through materials such as cloth, paper or rope can be used to purify water by transferring water through the wick material from a "cloudy" source into a clean container.
Below are suggested procedures, which you may need to alter, depending on student level, time constraints, and availability of materials.
Before the Activity
- Gather materials and organize lab stations.
- Make copies of Capillary Action Activities Worksheet.
With the Students
- Divide the class into lab groups and send them to the lab stations.
- Direct students to follow the worksheet lab instructions, recording data and answering questions.
- Part I: The Meniscus
- Fill a graduated cylinder with water.
- Observe, sketch and describe the meniscus.
- Answer questions to show understanding of the phenomena.
- Part II: Capillary Tubes

Figure 2: The height water rises in a capillary tube depends on the diameter of the capillary tube.
- Attach capillary tubes to a ring stand so that they are all suspended just over the bottom of a Petri dish (see Figure 2).
- Fill the Petri dish with water colored with a dark food coloring.
- Observe the movement of water up the capillary tubes and measure the final heights.
- Part III: Using Capillary Action
- Use the materials provided to design a capillary siphon. The finished length must be no longer than 30 cm and weigh no more than 100 g. Sketch your design:
- When finished building the siphon, use a piece of string to attach a team name label to the siphon and bring the siphon to the front table to be measured for meeting requirements.
- At the end of class, drape each siphon over two buckets, one full and one empty.
- The next day, at the beginning of class, have each group measure how much water transferred between the two buckets by capillary action through the siphon.
- Have each group record their findings on the board along with brief descriptions of their siphons.
- Have students complete the final worksheet question: Looking at the results from each group, can you draw any conclusions about which materials or designs worked the best?
- Assign students to teach what they have learned in the lab activities to a family member after school that evening, as described in the Assessment section. Follow-up in a later class period to discuss as a class how this went, how well they understood capillary action when teaching someone else, and any questions they could not answer.
Assessment
Pre-Activity Assessment
Brainstorming: Brainstorming: In small groups, have groups each place a water droplet on a table top and then place a piece of paper towel on top of it. Observe what happens. As a group, discuss how the water moves into the paper towel.
Activity Embedded Assessment
Worksheet: Students demonstrate their thought processes during the activity by answering the questions on the Capillary Action Activities Worksheet. They extend their learning by experiencing a real-world engineering application of capillary action by designing and using "capillary siphons" that can be used to filter water. Gauge student understanding by circulating the classroom and asking students how they answered specific worksheet questions.
Post-Activity Assessment
Teach What You Have Learned: Assign students to show a family member or a friend one or more of the lab activities. To prepare for this, after the lab has been turned in but before the end of class, have students each choose one lab activity and try to write out a procedure for it from memory. Check each written procedure briefly before students leave class. That evening, students explain to friends or family members what capillary action is and how it is working in the lab activity. During a later class period, discuss as a class how well they understood capillary action afterwards and any questions they could not answer.
Safety Issues
- Have students wear eye protection since glass is used in this experiment.
- Handle the capillary tubes with care! The tubes used in this experiment have a thick outer wall, but the glass is still fragile and can be easily broken if not treated gently.
Troubleshooting Tips
For Part 1: The Meniscus: Smaller graduated cylinders and plastic graduated cylinders both create more noticeable menisci.
For Part II: Capillary Tubes:
- Since capillary tubes are very fragile, depending on students and time constraints, you may want to prepare the capillary tubes and Petri dish set-ups (without water) before class.
- To best see the dye in the glass capillary tubes, make sure the water is dyed very dark with food coloring.
- Sometimes the height the water reaches in a capillary tube depends on which side is at the bottom; this may be due to variations in tube diameter.
- Clean capillary tubes as soon as possible after class by submersing them in water and then blowing air through them.
Activity Extensions
As an extension to this lab, have students use paper chromatography to separate the colors in marker or plant leaves and flowers (see Figure 4). Have students investigate how chromatography, based on capillary action, is used in various fields. For more information, see "Plant Pigment Chromatography" by W. Benya, listed in the References section.
Additional Multimedia Support
Show students another demonstration of capillary action in action by placing a wilted celery stalk in a glass of water with dark food coloring for a few days and watch for the movement of the color to the top leaves of the celery. See instructions and photos at the US Geological Survey's Water Science for Schools: Capillary Action website at: https://water.usgs.gov/edu/capillaryaction.html.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are presented with a short lesson on the difference between cohesive forces (the forces that hold water molecules together and create surface tension) and adhesive forces (the forces that causes water to "stick" to solid surfaces. Students are also introduced to examples of capillary action...

Students explore the basic characteristics of polymers through the introduction of two polymer categories: thermoplastics and thermosets. During teacher demos, students observe the unique behaviors of thermoplastics.

Student teams are challenged to evaluate the design of several liquid soaps to answer the question, “Which soap is the best?” Through two simple teacher class demonstrations and the activity investigation, students learn about surface tension and how it is measured, the properties of surfactants (so...

Students observe capillary action in glass tubes of varying sizes. Then they use the capillary action to calculate the surface tension in each tube. They find the average surface tensions and calculate the statistical errors.
References
Benya, W. "Plant Pigment Chromatography." Milan Area Schools. Accessed July 2010. (Detailed instructions for plant chromatography.) http://www.milan.k12.mi.us/~wbenya/Biology/documents/chromatography%20lab.pdf.
"Emergencies: Home Water Storage and Emergency Disinfection Before an Emergency." Last updated February 10, 2011. Utah Department of Environmental Quality. Accessed April 2011. (Description of capillary siphons.) http://www.drinkingwater.utah.gov/emergency_water_storage.htm.
"Paper Chromatography." Jchemed.chem.wisc.edu. Accessed September 2, 2010. (Brief explanation of paper chromatography with links to more information.) http://jchemed.chem.wisc.edu/JCEsoft/Programs/VideoCD/CPL/Sample/Modules/paprchrom/paprchromdesc.htm.
Robinson, Clay. "Capillary Action." Last updated January 27, 2009. West Texas A&M University. Accessed June 2009. (Basis for capillary tube lab experiment, with modifications.) http://www.wtamu.edu/~crobinson/SoilWater/capillar.html.
Copyright
© 2013 by Regents of the University of Colorado; original © 2010/11 Duke UniversityContributors
Jean Stave, Durham Public Schools, NC; Chuan-Hua Chen, Mechanical Engineering and Material Science, Pratt School of EngineeringSupporting Program
NSF CAREER Award and RET Program, Mechanical Engineering and Material Science, Pratt School of Engineering, Duke UniversityAcknowledgements
This digital library content was developed under an NSF CAREER Award (CBET- 08-46705) and an RET supplement (CBET-10-09869). However, these contents do not necessarily represent the policies of the National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: March 4, 2021






User Comments & Tips