Quick Look
Grade Level: 6 (5-7)
Time Required: 45 minutes
Expendable Cost/Group: US $6.00
Group Size: 2
Activity Dependency: None
Subject Areas: Biology, Life Science
NGSS Performance Expectations:

| MS-PS1-2 |
Summary
Students learn the function of the liver and how biomedical engineers can use liver regeneration to help people. Students test the effects of toxic chemicals on a beef liver by adding hydrogen peroxide to various liver and salt solutions. They observe, record and graph their results.Engineering Connection
Tissue and organ regeneration is one of the most cutting-edge engineering applications, and an area of significant engineering research. In labs across the world, mechanical and biomedical engineers are exploring and experimenting with how to "grow" artificial ligaments, organs, skin and spinal disks. Since human livers are able to regenerate, bioengineers can create new and functional organs using only a portion of another liver. In the future, instead of waiting for liver donations that match patients' blood types, hospitals may supply patients who have liver failure with whole liver organs ready for surgical replacement.
Learning Objectives
After this activity, students should be able to:
- Explain how different salt concentrations affect the liver's ability to break down toxins.
- Explain that the liver is the only internal organ that can currently be regenerated by biomedical engineers.
- Explain why tissue regeneration by biomedical engineers is important to human health.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS1-2. Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Analyze and interpret data to determine similarities and differences in findings. Alignment agreement: Science knowledge is based upon logical and conceptual connections between evidence and explanations.Alignment agreement: | Each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. Alignment agreement: Substances react chemically in characteristic ways. In a chemical process, the atoms that make up the original substances are regrouped into different molecules, and these new substances have different properties from those of the reactants.Alignment agreement: | |
Common Core State Standards - Math
-
Represent real world and mathematical problems by graphing points in the first quadrant of the coordinate plane, and interpret coordinate values of points in the context of the situation.
(Grade
5)
More Details
Do you agree with this alignment?
-
Represent and interpret data.
(Grade
5)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Advances and innovations in medical technologies are used to improve healthcare.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Colorado - Math
-
Represent and interpret data.
(Grade
5)
More Details
Do you agree with this alignment?
-
Represent real world and mathematical problems by graphing points in the first quadrant of the coordinate plane, and interpret coordinate values of points in the context of the situation.
(Grade
5)
More Details
Do you agree with this alignment?
Colorado - Science
-
Develop and design a scientific investigation about human body systems
(Grade
7)
More Details
Do you agree with this alignment?
-
Gather, analyze, and interpret data and models on the functions and interactions of the human body
(Grade
7)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- 3 test tubes
- 2 eye droppers (for salt solution and hydrogen peroxide)
- Stopwatch or timer
- 2 small plastic or glass containers to hold the salt solution and hydrogen peroxide (such as baby food jars; no lids required)
- Living with Your Liver Worksheet, one per student
- (optional) 3 12-inch (.3 m) balloons (see Troubleshooting Tips section)

For the entire class to share:
- Liver solution: In a blender, blend 1/4 pound (113 g) of diced fresh beef liver with 13.5 fluid ounces (104 ml) water. Keep the liver solution refrigerated until 20 minutes before the activity. Do not allow the mixture to be kept at room temperature.
- 1 eye dropper (for dropping liver solution into test tubes)
- 2% salt solution: Mix 0.07 ounces (2 g) of salt with 6.8 fluid ounces (200 ml) of tap water
- 3% hydrogen peroxide (H2O2; available at pharmacy or grocery store)
- (optional) Masking tape and markers (to label small jars and test tubes)
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_liver_activity1] to print or download.Pre-Req Knowledge
Students should know that the liver is the largest internal organ in the human body and understand that its function is to remove wastes and toxins, and store vitamins.
Introduction/Motivation
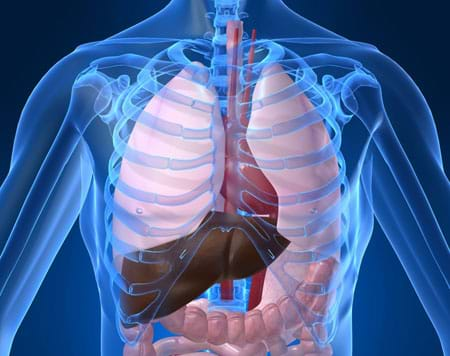
The liver is the largest internal human organ and is essential for human life. Your liver is located on the right side of your body beneath the diaphragm, which is just beneath your lungs. Feel the lower ribs on the right side of your body — your liver is just on the other side of those ribs! Most adults have a liver weighing 3 to 3.5 pounds (1.4 to 1.6 kg) — this weighs about the same as four cans of soda! Also, the liver takes up a lot of space — adult livers are about the size of a football!
The liver is the most important organ involved in production and storage of biochemicals. This organ detoxifies the body, removes bacteria and stores a lot of vitamins. Sometimes the liver is exposed to toxins for so long that it cannot perform its job. This is the case with cirrhosis, which can be caused by drinking too much alcohol for a decade or more. After encountering too many toxins for too long, the liver can slowly die. Sodium (salt) decreases the liver's ability to break down harmful toxins, such as hydrogen peroxide, which can be present in the body as a natural byproduct of cellular detoxification of other materials. Some studies link high-sodium diets to high blood pressure and heart disease.
By removing wastes (unneeded chemicals) and toxins, and storing vitamins, the liver helps us stay healthy. Sometimes, however, liver tissue is damaged and can die. Diseases such as alcoholism and Hepatitis C, for example, can cause serious liver damage. In severe cases, liver transplantation surgery may be necessary.
Tissue engineering is an area of research that combines engineering with life science. Tissue and organ regeneration is an area of significant engineering research. In labs across the world, mechanical and biomedical engineers are exploring and experimenting with how to "grow" artificial ligaments, organs, skin and spinal disks. Engineers study materials and artificial systems that can substitute for or strengthen damaged organ tissue in an organism. Tissue engineering involves the use of living cells in combination with an artificial support structure. Some materials used for support structures might include collagen or specialized polyesters. It is important for engineers to think about the different constraints that affect the choice of materials they use for tissue engineering. Can you think of some things that engineers might need to consider? Some material characteristics to consider might include replacement tissue size, material cell size, support structure size, rate of diffusion and degradation (how fast the material breaks down) within the human body.
The liver is one of the few human organs that can be regenerated. This means that in a lab we can generate an entirely new liver by using just a portion of a liver. In fact, using as little as one-quarter of an original liver is enough to create a whole new organ. Can you think of reasons why regenerating new organs may be useful? In the future, patients who suffer from liver failure may not need to wait for liver donations that match their blood types. Instead, hospitals may have functioning organs available ready to use! So you can see that it is important to understand the function of the liver if engineers are going to be successful in helping to protect and repair this vital human organ.
Procedure
Background Information
The liver's function is to process and remove waste and toxins. One of the cellular organelles, called peroxisomes, is responsible for detoxifying waste products or foreign toxins within the cell. Peroxisome naturally produces hydrogen peroxide (H2O2) during this detoxification process. If this hydrogen peroxide were allowed to build up, it would be harmful to the body. Found abundantly in the liver cells is an enzyme responsible for decomposing the H2O2 into harmless reagents, water (H2O) and oxygen (O2).
When encountering hydrogen peroxide, liver cells chemically break it into water and oxygen gas (2 H2O2 >> 2 H2O + O2). Because this reaction releases gas (which we know is oxygen because it is the only gaseous byproduct of the reaction), we can conclude that the liver solution is breaking down the hydrogen peroxide -- and is performing its job! When we add salt (and the same amount of hydrogen peroxide), however, the reaction gives off less gas (produces less oxygen). Therefore, the liver cells are less successful in breaking down the hydrogen peroxide; hence, the liver performs its job less effectively when salt is present.
Before the Activity
- Prepare the liver solution.
- Using the eye droppers, place 15 drops of the prepared liver solution into the test tubes. Make enough for each group to have three test tubes with 15 drops of the liver solution in each.
- Prepare the salt solution.
- Divide the prepared salt solution into smaller containers to distribute. Provide each group with one container of salt solution. Label the salt solution so it is not confused with the hydrogen peroxide.
- Divide the hydrogen peroxide into smaller containers to distribute. Provide each group with one container of hydrogen peroxide. Label the hydrogen peroxide so it is not confused with the salt solution.
- Make copies of the Living with Your Liver Worksheet, one per student.

With the Students
- Review the functions of the liver with students. Explain that hydrogen peroxide (H2O2) is a toxin that the liver breaks down to remove it from the body. Hydrogen peroxide can be present in the body as a natural byproduct of cellular detoxification of other materials. Be sure students understand that this reaction produces bubbles as the toxin is altered into other substances.
- Divide the class into teams of two students each. Distribute materials to each group (test tube with 15 drops of liver solution, salt solution, hydrogen peroxide, eye droppers and worksheets).
- Have each group decide how many drops of salt solution (between 0 and 30) they will add to each test tube. Remind the students that a wider range provides more interesting results. (Optional: Use tape and markers to identify how many drops will be placed in each test tube.)
- Tell students to add their specified number of drops of salt solution to each test tube of liver solution. Remind students to record these values on their worksheets.
- With timers ready, have students add 15 drops of hydrogen peroxide to one of their test tubes. Add the drops relatively quickly. Have students time how long it takes the liver solution to react. Remind students to record their observations about this reaction.
- Repeat step 4 for the second test tube. Remind students to compare and note the differences between this reaction and the previous.
- Repeat step 4 for the third test tube. Remind students to compare and note the differences between this reaction and the previous two.
- Have students finish the activity by completing the worksheet questions. (Optional: Have students graph their data results on the back of their worksheets, comparing number of salt drops versus time of reaction. See post-activity assessment on graphing in the Assessment section.)
- Conclude by leading students in a discussion drawn from their worksheet answers, as well as post-activity questions and answers provided in the Assessment section.
Vocabulary/Definitions
bioengineering: The use of artificial tissues, organs or organ components to replace damaged or absent parts of the body, such as artificial limbs, and heart pacemakers. Bioengineering combines biology and engineering.
biomedical engineer: An engineer who works closely with biologists and medical doctors to develop medical instruments, artificial organs, and prosthetic devices.
biomedical engineering: The application of engineering techniques to the understanding of biological systems and the development of therapeutic technologies and devices. Kidney dialysis, pacemakers, synthetic skin and organs, artificial joints, and prostheses are some products of biomedical engineering. Also called bioengineering.
cirrhosis: A chronic disease of the liver characterized by the replacement of normal tissue with scar tissue and the loss of functional liver cells. It is most commonly caused by chronic alcohol abuse, but can also result from nutritional deprivation or infection, especially by the hepatitis virus.
collagen: A type of protein found in cells, which forms fibers and is very strong in tension.
constraint: A limitation or restriction. For engineers, constraints are the limitations and requirements that must be considered when designing a workable solution to a problem.
liver: A large glandular organ in the abdomen of vertebrate animals that is essential to many metabolic processes. The liver secretes bile, stores fat and sugar as reserve energy sources, converts harmful substances to less toxic forms, and regulates the amount of blood in the body.
organelles: The "little organs" inside our bodies' cells. Examples cell membrane, cell wall, ribosomes, nucleus, mitochondria, ribosomes, endoplasmic reticulum, golgi apparatus and lysosomes.
polyester: A type of polymer made of natural and synthetic chemicals. Used to make fabric, pillow stuffing, seatbelts, or a strengthener for damaged tissue.
regeneration: The ability to grow or replace lost or damaged tissue.
toxin: A substance that is poisonous to the body. Can be products or byproducts of ordinary metabolism (wastes) that are not broken down or excreted before building up to dangerous levels in the body.
Assessment
Pre-Activity Assessment
Prediction: Have students predict the outcome of the activity before the activity is performed.
- What will happen to the liver's ability to function (break down hydrogen peroxide) when salt is added to the liver?
Question/Answer: Ask students questions and have them raise their hands to answer.
- What function does the liver perform? (Answer: The liver is responsible for removing waste materials and toxins, and storing vitamins in the human body.)
- Biomedical engineers have determined that the liver is one of the few organs that can be regenerated. What does this mean? (Answer: Regeneration is the ability to re-grow lost or damaged cells.)
- What might happen if a liver stops functioning? (Answer: If a liver stops functioning properly or encounters too many toxins it can die. In this case, a person needs a liver transplant to help his/her body regulate the wastes and toxins, and acquire essential vitamins.)
Activity Embedded Assessment
Worksheet: Have students complete the Living with Your Liver Worksheet. Review their data table and answers to gauge their understanding of the concepts.
Post-Activity Assessment
Graphing: Have students graph their results on the back of their worksheets, comparing number of salt drops versus time of reaction. How does the amount of salt affect the rate of reaction? Based on the graph, what would happen to the rate of reaction if you added more salt? Less salt?
Class Discussion: Have students raise their hands to share their answers to the worksheet questions. Talk about how salt affects the liver's ability to break down the toxin hydrogen peroxide. (Point to make: When salt was added to the liver, its ability to function [break down the hydrogen peroxide] was reduced.) Ask students to give advice to someone who has a very high salt content in his/her diet. (Example advice: Because a diet high in salt reduces the liver's ability to break down toxins, it is important to consume moderate amounts of salty foods). Point out that excessive alcohol can cause similar harmful effects to the liver's ability to break down toxins. Ask students to think about how salt might affect a biomedical engineer's development of tissue replacement processes. (Possible examples: Biomedical engineers may want to develop liver tissue that can withstand high salt content.) What types of things might an engineer need to consider when developing replacement liver tissue for a person with high salt content in their environment? (Possible considerations: Material characteristics such as replacement tissue size, material cell size, support structure size, rate of diffusion and degradation [how fast the material breaks down] within the human body.)
Safety Issues
- Although 3% hydrogen peroxide is relatively innocuous, it is important to follow basic safety precautions when handling chemicals. Use the hydrogen peroxide in a well-ventilated area; concentrated fumes may irritate the sinuses and eyes. Be careful to keep hydrogen peroxide away from the eyes and mouth. Also avoid contact with clothing.
- It is safe to dispose of liver, liver solution, salt solution, and hydrogen peroxide down the lab sink drain. Rinse the sink area well with warm water and soap to avoid odors. Alternatively, pour these items into a container (such as a glass jar or plastic bottle) and dispose in a trash receptacle.
Troubleshooting Tips
If the difference between reaction rates is difficult to discern, try placing a standard 12-inch balloon over the test tube after adding the hydrogen peroxide. The gas produced by the chemical reactions fills the balloon. For the lower salt content solutions, the balloon should expand more than for the higher salt content solutions.
Activity Extensions
Have students research liver regeneration. What is liver generation? In what ways do engineers think it may help people?
Have students research the range of different materials used in tissue engineering. What are the latest developments in tissue engineering?
Have student research why tissue culture is important in tissue engineering. What types of specification might a tissue culture have? What types of devices do engineers design to aid in tissue cultures?
What other engineering innovations help people with liver problems? Direct students to research liver dialysis and bioartificial livers. How do they work?
Activity Scaling
- For lower grades, conduct the experiment as a classroom demonstration. Have students sit close enough to observe the differences between the tests. Use the Living with Your Liver Worksheet (for Lower Grades).
- For upper grades, have students research the technology of organ regeneration to develop an understanding of what organ regeneration is and what engineers are doing to evolve this technology. Require students to turn in a one-page summary of what they learned.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

This lesson helps students explore the functions of the kidney and its place in the urinary system. Students learn how engineers design instruments to help people when kidneys are not functioning properly or when environmental conditions change, such as kidney function in space.
References
Bio/Biomedical Engineering. The Engineering Alphabet, ASEE Engineering K12Center, American Society for Engineering Education. egfi-k12.org Accessed July 16, 2008.
Bioengineering. 2008. The Oxford Pocket Dictionary of Current English. www.encyclopedia.com Accessed July 16, 2008.
Cirrhosis. Dictionary.com. The American Heritage Dictionary of the English Language, Fourth Edition. Houghton Mifflin Company, 2004. dictionary.reference.com Accessed July 16, 2008.
Liver. Dictionary.com. The American Heritage Dictionary of the English Language, Fourth Edition. Houghton Mifflin Company, 2004. dictionary.reference.com Accessed July 16, 2008.
Copyright
© 2008 by Regents of the University of Colorado.Contributors
Megan Schroeder; Malinda Schaefer Zarske; Denise W. CarlsonSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: April 20, 2019


User Comments & Tips