Quick Look
Grade Level: 5 (4-6)
Time Required: 15 minutes
Expendable Cost/Group: US $15.00
Group Size: 28
Activity Dependency: None
NGSS Performance Expectations:

| 5-ESS3-1 |
Summary
Students observe and discuss a simple model of a wet scrubber to understand how this pollutant recovery method functions in cleaning industrial air pollution.Engineering Connection
One of the biggest challenges environmental engineers face is devising new techniques to prevent industrial air pollution. Engineers are creative in designing modern pollutant recovery methods and industrial technologies that clean up and prevent air pollution. Scrubbers are designed for use at power plants and facilities that emit sulfur dioxides and hydrogen sulfide gases. Engineers also design cleaning products that scrub stains from your tub and shower, and non-toxic technologies that clean your oven.
Learning Objectives
After this activity, students should be able to:
- Describe and explain how a wet scrubber works as a pollutant recovery method.
- Give examples indicating when the use of a wet scrubber is appropriate.
- Describe how engineers create technology to help industry clean up their air pollution.
- Use their knowledge of percentages to understand technological efficiency.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
5-ESS3-1. Obtain and combine information about ways individual communities use science ideas to protect the Earth's resources and environment. (Grade 5) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Obtain and combine information from books and/or other reliable media to explain phenomena or solutions to a design problem. Alignment agreement: | Human activities in agriculture, industry, and everyday life have had major effects on the land, vegetation, streams, ocean, air, and even outer space. But individuals and communities are doing things to help protect Earth's resources and environments. Alignment agreement: | A system can be described in terms of its components and their interactions. Alignment agreement: Science findings are limited to questions that can be answered with empirical evidence.Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the effects of technology on the environment.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Explain why responsible use of technology requires sustainable management of resources.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
Materials List
For the class demonstration:
- Duct tape
- 1/2 cup chalk dust or fireplace ash
- 3 clear, glass bottles (such as large olive oil or liquor bottles)
- 3 rubber corks with 2 holes drilled in each (the corks must fit snuggly into the bottle openings; available at hardware stores, sometimes predrilled)
- Various pipe connectors, as needed, to transition the narrow tubing to the wider vacuum cleaner nozzle (see Figure 2)
- 5 barb connectors (to connect plastic tubing to corks; the connector must fit snuggly into the holes in the corks; available at hardware stores; see Figure 3)
- 5 ft. clear plastic tubing (the tubing must fit snuggly over the barb connectors; available at hardware stores; see Figure 3)
- Water
- Paper towels
- Wet/dry shop vacuum cleaner

Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_air_lesson10_activity1] to print or download.Pre-Req Knowledge
A basic understanding of air pollution.
Introduction/Motivation
One of the biggest challenges environmental engineers face is devising new techniques to prevent additional air pollution. Industry is a large contributor to air pollution. Have you ever seen a big factory releasing dark smoke into the air? How does it smell? Sometimes, it does not smell so great! The first type of technology (or pollutant recovery method) we are going to examine is a wet scrubber. A wet scrubber uses water to clean pollutants out of the air.
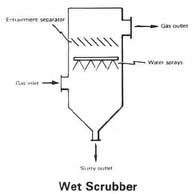
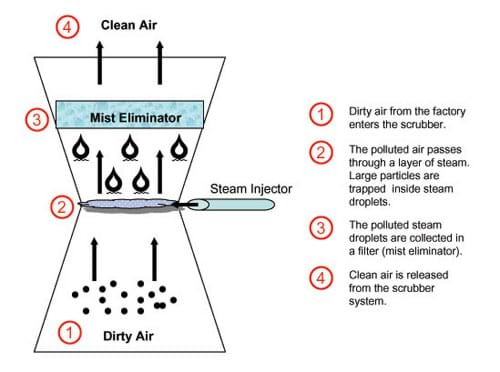
Scrubbers are used at coal burning power plants, asphalt/concrete plants, and a variety of other facilities that emit sulfur dioxides, hydrogen sulfide and gases with high water solubility. Inside a wet scrubber, solid particles and gases are trapped as they pass through a fine water mist (refer to Figure 1 or the attached How a Wet Scrubber Cleans Polluted Air Diagram). Sometimes the mist is injected with limestone powder to help extract the dirt particles. Wet scrubbers are often used for corrosive, acidic or basic gas streams. The resulting pollutant-laden wastewater is processed into sludge, which is used to make bricks.
How well do you think this works? Today, we are going to model the wet scrubber technology in class.
Procedure
Before the Activity
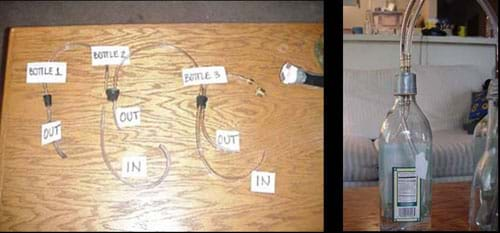
- Gather materials and set up the apparatus as shown in Figures 3 and 4. Refer to the Wet Scrubber Model Photo Collection (attachment) for more details.
- Test the activity at least once before presenting it as a class demonstration.

With the Students
- As a class, brainstorm different ways that water could be used to clean the air. List ideas on the board.
- Put chalk dust or fireplace ash (representing pollution) in the first bottle.
- Fill bottle 2 and bottle 3 both 1/3 full of water.
- Make sure the "in" tube in each bottle is submerged in the water, while the "out" tube is not.
- Connect the "out" tube of bottle 3 to the shop vacuum cleaner. Use the other pipe connectors (see Figure 2) to make a tight connection from the narrow tube to the larger vacuum cleaner tube. Use duct tape to make all the connections airtight.
- When ready to start, shake bottle 1 to create the polluted air and turn on the vacuum cleaner at a low setting.
- What should happen: Polluted/dirty air in bottle 1 is suctioned into the water in bottle 2. The bottle 2 water cleans the particulates from the air while the cleaner air is suctioned on into bottle 3. Dust may collect in the air above the water in bottle 2. This is why there is a third bottle. The air is cleaned a second time in bottle 3 before being suctioned out by the vacuum cleaner as clean air released into the environment.
- Have students record their observations. Tell them to write down anything that strikes them as important.
- Discuss the student observations. Explain that a wet scrubber collects not only particulate matter but also captures waste gases. Discuss that the white plume you see coming from a smokestack of a factory may really be (unpolluted) steam coming from a water scrubber.
- In conclusion, ask the students the following questions, and discuss as a class:
- Why does the water in the wet-scrubber change color? (Answer: Visible air pollution [particulate matter] moves from the air into the water.)
- What does the scrubber filter out of the air? What does it not filter out? (Answer: It filters out fine particulate matter and waste gases. It does not filter out heavy particles of dirt and sludge.)
- Suggest ways to dispose of the pollutants that are now trapped in the water. (Possible answer: The resulting pollutant-laden wastewater is processed into sludge, which is used to make bricks.)
- Does the wet scrubber remove all of the particulates? (Answer: The average efficiency of a wet scrubber is about 95%. That means that if there are 100 particulates, 95 of them are caught and six of them make it back into the air.)
- Is this a good or bad efficiency rate? (Opinions may vary. 95% still means that 5% of the particulates are returning to the atmosphere.)
Assessment
Pre-Activity Assessment
Brainstorming: As a class, have the students engage in open discussion. Remind students that in brainstorming, no idea or suggestion is "silly." All ideas should be respectfully heard. Take an uncritical position, encourage wild ideas and discourage criticism of ideas. Have them raise their hands to respond. Write their ideas on the board. Ask the students:
- How can water be used to clean the air?
Activity Embedded Assessment
Observations: Ask students to pay close attention and record their observations of the experiment, explaining to them that this is what real scientists and engineers do. Instruct them to record anything that seems important.
Post-Activity Assessment
Observations: Discuss student observations.
Drawing/Journaling: Depending on the students' age, have them draw a picture or write in their own words a description of how a wet scrubber works to clean polluted air. Ask for volunteers to share their descriptions with the class.
Safety Issues
- Only draw air through the tubing using a vacuum cleaner; using your mouth may cause accidental inhaling of the contaminated air.
Troubleshooting Tips
The corks, tubing and connectors can be purchased at a hardware store. It is possible to find the corks with predrilled holes.
Make sure that all the connections are tightly sealed with duct tape.
If you are using fireplace ash, replenish it frequently because only the light "floaty" particulates are pulled through the scrubber by the vacuum; the heavier charcoal pieces are not useful for the demonstration.
It is strongly recommended that you practice this demonstration at least once before presenting it to the students.
Activity Extensions
A wet scrubber's resulting pollutant-laden wastewater is sometimes processed into sludge, which is used to make bricks. How does this work? Have students investigate this process on the Internet (search: sludge dryers, dewatering sludge) and report back to class. Is this a successful pollutant recovery process?
Find local examples of wet scrubbers and arrange a field trip. What pollutants does this wet scrubber remove? What do they do with the wet scrubber wastewater?
Activity Scaling
- While the demonstration is appropriate for any age level, younger students may have difficulty understanding some of the ways the scrubber functions. Tailor the explanation and discussion to fit the comprehension level of your students.
- For upper grades, add a math component. Give students a number of particulates, and have them calculate how many are cleaned from the air using a wet scrubber with 95% efficiency. For example, if 100,000 particulates went through this wet scrubber, about 100,000 x 0.95 = 95,000 would be removed, and 5,000 would remain.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

To develop an understanding of modern industrial technologies that clean up and prevent air pollution, students build and observe a variety of simple models of engineering pollutant recovery methods: scrubber, electrostatic precipitator, cyclone and baghouse. In an associated literacy activity, stud...

Students are introduced to the concepts of air pollution and technologies that engineers have developed to reduce air pollution. They develop an understanding of visible air pollutants with an incomplete combustion demonstration, a "smog in a jar" demonstration, construction of simple particulate ma...

Students are introduced to the concepts of air pollution, air quality, and climate change. The three lesson parts (including the associated activities) focus on the prerequisites for understanding air pollution. First, students use M&M® candies to create pie graphs that express their understanding o...

Students learn what causes air pollution and how to investigate the different pollutants that exist, such as toxic gases and particulate matter. They investigate the technologies developed by engineers to reduce air pollution.
References
Foresman, Scott. Science Insights – Exploring Matter and Energy. Reading, MA: Addison Wesley, 1999.
Markle, Sandra. The Kids' Earth Handbook. Atheneum, NY: John Wiley & Sons, Inc, 1991.
Copyright
© 2004 by Regents of the University of Colorado.Contributors
Amy Kolenbrander; Sharon Perez; Janet Yowell; Natalie Mach; Malinda Schaefer Zarske; Denise CarlsonSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: August 31, 2020






User Comments & Tips