Quick Look
Grade Level: 6 (4-6)
Time Required: 45 minutes
Lesson Dependency: None
NGSS Performance Expectations:

| MS-ESS3-3 |
Summary
Students develop an understanding of visible air pollutants with an incomplete combustion demonstration, a "smog in a jar" demonstration, building simple particulate matter collectors, and exploration of engineering roles related to air pollution. In an associated literacy activity, students learn basic marketing concepts and techniques, and the principles of comparative analysis, while creating an advertisement for a hybrid vehicle. Note: You may want to set up the associated activities simultaneously as they require extended data collection time and can share test sites.Engineering Connection
Engineers continually work to prevent smog and visible particulate matter pollution so our air is safe to breathe. One mechanical engineering invention is a catalytic converter that reduces smog by changing hydrocarbons and carbon monoxide in automobile exhaust into less harmful carbon dioxide and water vapor. Engineers continue to explore new, creative ideas to lower the emissions into the air, such as designing more efficient vehicles and filters to reduce the amount of particulate matter released into the atmosphere by the machinery and manufacturing processes of our modern world.
Learning Objectives
After this lesson, students should be able to:
- Identify the two major types of visible pollutants, smog and particulate matter.
- Understand and explain how air pollutants are generated.
- Understand and explain how smog forms.
- Describe how engineers interact with visible air pollution.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-ESS3-3. Apply scientific principles to design a method for monitoring and minimizing a human impact on the environment. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Apply scientific principles to design an object, tool, process or system. Alignment agreement: | Human activities have significantly altered the biosphere, sometimes damaging or destroying natural habitats and causing the extinction of other species. But changes to Earth's environments can have different impacts (negative and positive) for different living things. Alignment agreement: | Relationships can be classified as causal or correlational, and correlation does not necessarily imply causation. Alignment agreement: The uses of technologies and any limitations on their use are driven by individual or societal needs, desires, and values; by the findings of scientific research; and by differences in such factors as climate, natural resources, and economic conditions. Thus technology use varies from region to region and over time.Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
The management of waste produced by technological systems is an important societal issue.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
-
Analyze how the creation and use of technologies consumes renewable and non-renewable resources and creates waste.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Colorado - Science
-
Identify evidence suggesting that atoms form into molecules with different properties than their components
(Grade
6)
More Details
Do you agree with this alignment?
-
Develop, communicate, and justify an evidence-based explanation about how ecosystems interact with and impact the global environment
(Grade
6)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Introduction/Motivation
Before the Lesson — Not Your Average Toe Cheese!
- (Optional) Create an air pollution bulletin board that includes definitions and examples of the vocabulary terms. See the Air Pollution Bulletin Board Example attachment.
- Prepare the sock(s). Place a white sock over the tail pipe of your car. Be sure the tail pipe is cool (the car should NOT have been running recently). Turn on your car and run it for a minute or so. Then turn the car off and wait a minute or so to let the tail pipe cool before removing the sock. This may take longer with newer cars. DO NOT breathe the car exhaust.
- Set up a designated area in your classroom (bulletin board, specific table, etc.) to display the sock with an attention-getting title such as "What is this?" or "It's not your average toe cheese!" Supply the area with the sock, a magnifying glass, voting ballots (use the Voting Ballots attachment) and a ballot box (a covered shoe box works great). Scaling idea: For less advanced students, adapt the ballots to have the students draw pictures of their observations.
With the Students
- During the course of the lesson (or a longer period of time) ask students to observe the sock with the magnifying glass.
- Ask them to write down (on their ballot) what they observe.
- Ask students to hypothesize where the "sock pollution" came from. Encourage them to support their guess with evidence from their observations.
Lesson Background and Concepts for Teachers
The atmosphere is almost completely composed of invisible gaseous substances. Some of these gases pollute the air. Although most major air pollutants are invisible, particulate matter and smog can easily be seen, often from many miles away, especially when large amounts concentrate in cities. Because visible pollutants are more obvious, we understand a lot about them and they are heavily regulated by the U.S. Environmental Protection Agency.
Particulate Matter

Have you ever noticed a sunbeam with millions of little specks of dust floating in it? That is particulate matter. Particulate matter, often visible in the air, is tiny particles of solid matter and/or droplets of liquid. Natural sources of particulate matter include pollen, volcanic ash and dust blown by the wind. The primary sources of man-made particulate pollutants are the coal and oil burned by factories and power plants, and the hydrocarbon fuels burned by vehicles.
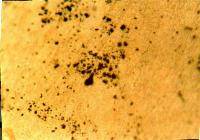
A close look at the particulate matter of air pollution, such as soot, car exhaust, ash and asbestos, reveals how different from each other these particulates look at the microscopic level. They vary greatly in size, shape and color. To show the students, use the Microscopic View of Air Pollutants attachment to make an overhead transparency or color print of some examples.
The U.S. has more than 150 million cars — think about how much pollution that creates! Other smaller-scale, but equally important, local sources of air pollution include the wood burned in fireplaces and wood-burning stoves. Some cities, such as Denver, CO, often have days when wood burning is restricted because of how much it contributes to poor air quality.
Particulate matter is harmful to people and animals who breathe it, and to the surfaces of buildings and other structures that are exposed to it for long periods of time or in large concentrations. For example, the Sistine Chapel in Rome, Italy, painted by Michelangelo, was dirtied over the centuries by air pollution from burning candles.
Smog
The word "smog" comes from the combination of the words "smoke" and "fog." It was first used in the early 1900s to describe the combination of smoke and thick fog that sometimes hung over the skies of London, England. Smog is a type of visible air pollution created from smoke, fog, suspended particles and chemical fumes. London-type smog comes from moisture in the air condensing on the smoke/chemical-suspended particles and forming tiny smog droplets. Refer to the associated activity Particulate Matter: For Your Eyes Only for students to develop their understanding of visible air pollutants with an incomplete combustion demonstration, a "smog in a jar" demonstration, and by building simple particulate matter collectors.

Weather conditions, such as a lack of wind or a thermal inversion, can cause smog to build up. Smog is especially obvious during a thermal inversion, when an upper layer of warm air traps the pollutants over a lower layer of cool air, preventing normal atmospheric circulation (the smog cannot rise and scatter). Mountain ranges near cities may also trap smog in an area.
There are two types of smog: photochemical smog and industrial smog. Photochemical smog occurs when strong sunlight reacts with air pollution such as the hydrocarbons and nitrogen oxides released from vehicles and power plants. The sun "bakes" the chemicals into the brownish-orange haze often seen in the skies above large cities. Low-level ozone is a major component of photochemical smog (do not confuse low-level ozone with upper-level ozone, which helps filter out harmful rays from the sun). Industrial smog contains sulfur oxide and solid particles that are released when fossil fuels are burned. It appears as a grayish haze in the skies above heavily industrialized cities.

Sometimes Hawaii has "vog" alerts — times when volcanically-produced smog is at dangerous levels.
What are Engineers Doing?
Engineers are continually working to help prevent smog and particulate matter pollution. One mechanical engineering invention is a catalytic converter that reduces smog by changing hydrocarbons and carbon monoxide in automobile exhaust into carbon dioxide and water vapor. While this is an improvement, high levels of carbon dioxide are still bad for human health and the environment so engineers continue to explore new ideas. One idea is to use energy from the sun — solar power — to run our cars and heat our homes. Another idea is to take advantage of the wind to create energy for use in factories, instead of burning coal or other fossil fuels. Students can play their part to spread the word with the literacy-based activity Green Marketing where they learn basic marketing concepts and techniques, and the principles of comparative analysis, while creating an advertisement for a hybrid vehicle.
Associated Activities
- Particulate Matter: For Your Eyes Only - Students develop their understanding of visible air pollutants with an incomplete combustion demonstration, a "smog in a jar" demonstration, and by building simple particulate matter collectors.
Lesson Closure
Class Discussion
- As a class, read and discuss the results of the student ballots.
- Ask the students if any of them would change the responses on their ballots based on what they learned during this lesson.
- Reveal the source of the sock pollution. (Answer: Directly from the tail pipe of your running car.)
- Discuss what actually comes out of cars' exhaust pipes, both visible and invisible. (Answer: carbon dioxide – CO2, carbon monoxide – CO, hydrocarbons and nitrogen oxides.)
- Where does the exhaust usually go? (Answer: Into the atmosphere.)
- Ask students to imagine millions of cars, right now, releasing pollution into the atmosphere. Remind them that engineers continue to work to improve fuels and engine efficiency to reduce these types of pollutants. Older cars, trucks and SUVs "give off" more pollution than newer cars.
- What are some ways engineers are reducing air pollution from cars? (Possible answers: Electric cars, hybrid gas and electric cars, increased fuel efficiency in vehicles.)
- How can you help engineers to reduce air pollution? (Possible answers: Ride your bicycle or walk instead of taking the car, recycle and reuse items so fewer items need to be produced in polluting factories.)
Vocabulary/Definitions
catalytic converter: An emission-control device that changes hydrocarbons and carbon monoxide in automobile exhaust into carbon dioxide and water vapor.
hydrocarbon: An organic compound containing atoms of hydrogen and carbon.
industrial smog: Contains sulfur oxide and solid particles that are released when fossil fuels are burned. It often appears as a grayish haze in the skies above heavily industrialized cities.
particulate matter: Very small particles of solid matter and/or droplets of liquid. It includes materials that have been burned (ash, smoke, soot), dust, pollen, soil or chemicals — anything that floats in the air.
photochemical smog: Appears as a brownish-orange haze often seen in the skies above large cities. Occurs when strong sunlight reacts with air pollution such as the hydrocarbons and nitrogen oxides released from vehicles and power plants. Ozone is a major component of photochemical smog.
pollutant: A harmful chemical or waste material that contaminates air, soil or water.
smog: A combination of smoke and fog in the air.
thermal inversion: When an upper layer of warm air traps the pollutants over a lower layer of cool air, preventing normal atmospheric circulation.
visible pollutant: Air pollution that can be seen with the naked eye.
Assessment
Pre-Lesson Assessment
Demonstration: Prepare for the Not Your Average Toe Cheese! classroom demonstration / activity as described in the Introduction/Motivation section.
Post-Introduction Assessment
Observation: Have students examine the sock and record their observations as directed in the Introduction/Motivation section.
Lesson Summary Assessment
Discussion: During lesson closure, have students identify which type of visible pollutants they found on the sock. (Note: In the lesson Invisible Air Pollution: I Don't Believe My Eyes!, students learn more about the invisible pollutants.)
Homework
Cartoon Character: Student assignment: Over the years, cartoon characters, such as Bugs Bunny, have often informed us about environmental issues. Create your own cartoon sketch of a character dealing with pollution. Your character could be modern or futuristic, a pollutant or a pollution fighter. Make sure to name your character and have it saying something about how the pollution is affecting them.
Internet Search: Assign students one vocabulary term each and have them research it on the Internet. Lead a small discussion of student findings during the next class period.
Lesson Extension Activities
Variations to the Not Your Average Toe Cheese! activity:
- Make more polluted socks and have groups of students observe them. As a class, discuss their ideas for the origination of the pollutants.
- Collect pollutants in the sock as a live demonstration in your school parking lot. Be sure the kids are on the sidewalk and that only an adult handles the sock and starts/stops the car.
- Compare the exhaust of different types (make and year) of cars. Does it make a difference? Conduct an emissions sock test before and after a car's tune-up.
Take a field trip to an emissions testing site. Or, invite a speaker from an emissions testing site to speak to your class about how they measure the vehicle exhaust pollutants. What determines whether a car passes or not? See the lesson I've Gotta Get Some Air, for information on how we clean up indoor air pollutants, and Pollution Solutions, for information on how we clean up outdoor air pollutants.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are introduced to the concepts of air pollution and technologies that engineers have developed to reduce air pollution. They develop an understanding of visible air pollutants with an incomplete combustion demonstration, a "smog in a jar" demonstration, construction of simple particulate ma...

Students are introduced to the concepts of air pollution, air quality, and climate change. The three lesson parts (including the associated activities) focus on the prerequisites for understanding air pollution. First, students use M&M® candies to create pie graphs that express their understanding o...

Students learn what causes air pollution and how to investigate the different pollutants that exist, such as toxic gases and particulate matter. They investigate the technologies developed by engineers to reduce air pollution.

Students learn the basics about the structure of the Earth’s atmosphere, the types of pollutants that are present in the atmosphere (primary, secondary, gas-phase compounds, particulate matter), and the importance of air quality research. They are also introduced to some engineering concepts such as...
References
Bosak, Susan V. Science is...: A Source Book of Fascinating Facts, Projects and Activities. Markham, Ontario: Scholastic Canada, Ltd., 1991. (Not Your Average Toe Cheese! Activity adapted from "Sock It to Me," pg. 362.)
Foresman, Scott. Science Insights – Exploring Matter and Energy. Boston, MA: Addison Wesley, 1999.
Maton, Anthea. Prentice Hall Science – Ecology Earth's Natural Resources. Upper Saddle River, NJ: Prentice-Hall, Inc., 1993.
Smog — Who Does It Hurt? What You Need to Know about Ozone and Your Health. July 1999. U.S. Environmental Protection Agency. www.epa.gov. Accessed October 19, 2004.
Stille, Darlene R. The New True Book – Air Pollution. Children's Press, Inc., 1990.
Copyright
© 2004 by Regents of the University of ColoradoContributors
Amy Kolenbrander; Janet Yowell; Natalie Mach; Malinda Schaefer Zarske; Denise CarlsonSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: December 11, 2020






User Comments & Tips