Quick Look
Grade Level: 5 (4-6)
Time Required: 45 minutes
Lesson Dependency: None
Subject Areas: Chemistry
NGSS Performance Expectations:

| 5-ESS3-1 |

Summary
Students explore the causes and effects of the Earth's ozone holes through discussion and an interactive simulation. In an associated literacy activity, students learn how to tell a story in order to make a complex topic (such as global warming or ozone holes) easier for a reader to grasp.Engineering Connection
Once our awareness was heightened about the human-created pollutants that cause the depletion of the Earth's protective ozone layer, engineers began to address the problem. They invented new technologies and/or re-designed old technologies to entirely avoid producing harmful chlorofluorocarbons (CFCs). Pervasive changes to design, manufacturing processes, regulations and practices have reduced U.S. CFC emissions dramatically. Some changes included new types of refrigerator coolants and the elimination of many aerosols. The modern engineer always keeps long-term sustainability in mind as a design objective.
Learning Objectives
After this lesson, students should be able to:
- Describe the benefits and disadvantages of ozone.
- Understand and explain the different processes that destroy ozone in the Earth's atmosphere.
- Develop an understanding of the global trend towards ozone depletion and recovery.
- Explain how engineers are trying to help reduce ozone depletion.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
5-ESS3-1. Obtain and combine information about ways individual communities use science ideas to protect the Earth's resources and environment. (Grade 5) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This lesson focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Obtain and combine information from books and/or other reliable media to explain phenomena or solutions to a design problem. Alignment agreement: | Human activities in agriculture, industry, and everyday life have had major effects on the land, vegetation, streams, ocean, air, and even outer space. But individuals and communities are doing things to help protect Earth's resources and environments. Alignment agreement: | A system can be described in terms of its components and their interactions. Alignment agreement: Science findings are limited to questions that can be answered with empirical evidence.Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Analyze how different technological systems often interact with economic, environmental, and social systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Colorado - Science
-
Identify evidence suggesting that atoms form into molecules with different properties than their components
(Grade
6)
More Details
Do you agree with this alignment?
-
Interpret and analyze data about changes in environmental conditions – such as climate change – and populations that support a claim describing why a specific population might be increasing or decreasing
(Grade
6)
More Details
Do you agree with this alignment?
Worksheets and Attachments
Visit [www.teachengineering.org/lessons/view/cub_air_lesson08] to print or download.Introduction/Motivation
The ozone layer is an important protective shield for life on Earth, filtering out more than 99% of the ultraviolet rays from the sun before they reach us. Ultraviolet rays cause sun tans and sun burns on our skin. The amount of ozone protecting us is decreasing. Some scientists predict that the increasing ultraviolet radiation that passes through our "shield" will increase the incidences of skin cancer, immune deficiencies and cataracts. In 1987, the U.S. Environmental Protection Agency (EPA) estimated that 40 million Americans will get skin cancer during the next 88 years and of those, 800,000 will die. Even more serious is the fact that the rate of ozone depletion, or the reducing of our protective shield, is increasing, and at a fast rate! Students can model this rate of depletion with the hands-on associated activity Gumdrop Ozone Depletion Model: Battling for Oxygen.
Ozone gas in the stratosphere forms when oxygen molecules interact with ultraviolet rays from the sun. The amounts of ozone in the stratosphere are changing all the time. Under normal circumstances, ozone is continuously being destroyed and regenerated by the sun's ultraviolet rays. The seasons of the year, changing winds and even sunspots affect ozone levels. Harmful pollutants in the air can also affect ozone by converting it into oxygen molecules and atoms, actually forcing ozone to break down more rapidly than it can rebuild.
Actual holes in the ozone protecting the Earth have been found. These "holes" are not completely empty of ozone, but the ozone concentrations in these areas are lower than under normal conditions, allowing more ultraviolet radiation to reach the Earth's surface. Ozone holes currently exist over Antarctica, the northern half of the U.S. into Canada, and northern Europe. Since scientists started measuring the ozone layer in the mid-1970s, it has become clear that the ozone layer is thinning even more quickly than first predicted. It is important to communicate this information in a way that is understandable. Students can use the literacy based associated activity Metamorphosis — Stories of Change to learn how to tell a story in order to make a complex topic (such as global warming or ozone holes) easier for a reader to grasp.
The only practical approach to stopping the destruction of the ozone layer is to reduce the amount of human-created pollutants that contribute to its depletion. Efforts to protect the ozone layer now involve many different nations and industries. The most common ozone-destroying pollutants are in a class of chemical compounds called chlorofluorocarbons (CFCs), which are used in air conditioner refrigerator coolants, lubricants, cleaning solvents, plastic foam manufacturing and aerosol spray propellants. Engineers are inventing and designing new technologies to help save the ozone and stop its reduction due to harmful CFCs.
For more in-depth information about ozone (or for advanced readers in your class), see the attached, two-page Ozone Reading.
Lesson Background and Concepts for Teachers
Paleobiologists (scientists who study fossils to learn about the Earth's ancient biology) tell us that just as life was emerging on Earth, the atmosphere would have been lethal to humans (little or no oxygen, but plenty of methane, ammonia, hydrogen sulfide, chlorine and carbon monoxide). At that time, there was no ozone layer to filter out the sun's ultraviolet radiation. By three billion years ago, chlorophyll developed, and began to use carbon and sunlight to make food, producing oxygen as a by-product. Some oxygen molecules combined to form ozone that created a protective ozone layer perhaps 600 million years ago. During millions of years, plants proliferated and produced oxygen at a much greater rate than it was consumed. About 350 million years ago, the atmospheric oxygen reached its present-day level of about 21%.
Ozone is a form of oxygen (with three oxygen atoms) that occupies most of the atmosphere between 10-30 miles above the Earth. When located at ground level and trapped near the Earth's surface, ozone is one of the most dangerous components of smog. In that situation, it cannot move higher in the atmosphere where it would filter out most of the sun's ultraviolet (UV) rays. Atmospheric ozone is a bluish gas about 12 miles (20 km) thick, found just inside the stratosphere (see Figure 1). It covers the Earth like a blanket and stops harmful light rays (most UV-B and all UV-C) from reaching us.
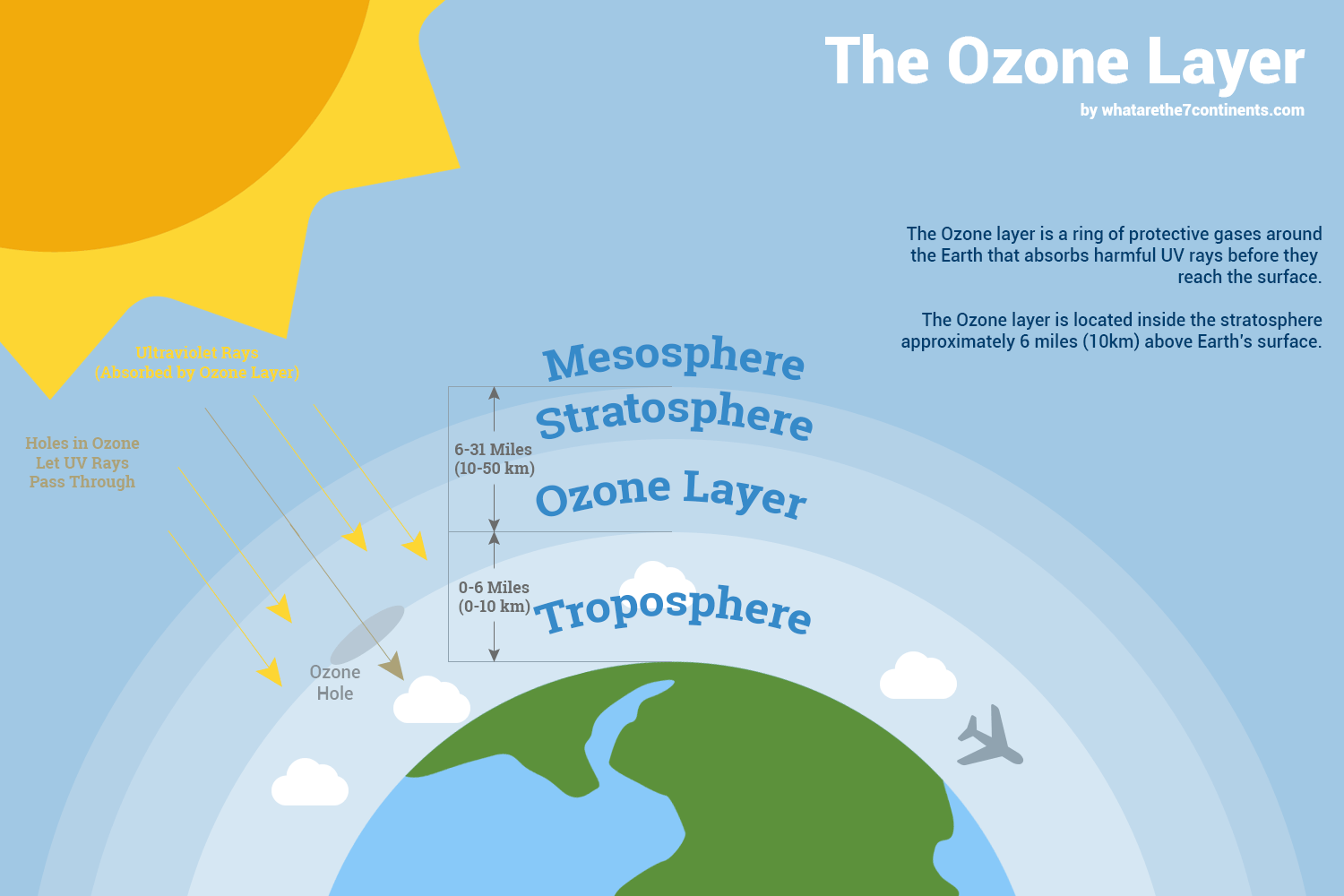
This type of UV radiation is known to weaken our immune systems, cause sunburns (tans), wrinkles, skin cancer and cataracts, and be harmful to plants (crop damage, by destroying germinating seeds), and life in the top layer of the oceans. Fortunately, most UV light is absorbed by the protective ozone layer.
The Earth's protective ozone layer starts about 10 miles (15km) above the Earth, extends to an altitude of about 30 miles (50km), and contains 90% of the Earth's ozone (the other 10% is at low altitudes and contributes significantly to air pollution). If all of the ozone in the atmosphere were subjected to the temperature and pressure at the Earth's surface, it would form a layer only 0.07 -0.19 inches (2-5 millimeters) thick!
The atmospheric ozone absorbs UV light according to the following process: A ray of UV light strikes an ozone molecule, is absorbed and transfers its energy to an individual oxygen atom, giving the atom enough energy to break free of the ozone molecule. Approximately 300 million tons of ozone is converted to oxygen and back to ozone every day through this process — which means a lot of UV light is being absorbed. This natural process was in balance, and the ozone layer maintained a steady thickness, until scientists in the 1960s noticed a thinning in the layer above Antarctica. This thinning became more pronounced every year, until it came to be known as the "ozone hole." This hole is not in fact a complete absence of ozone, but it is nearly half the thickness of the original layer.
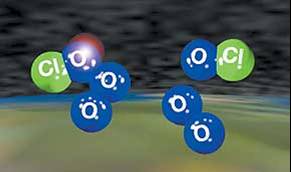
In the early 1970s, it was discovered that chlorine atoms in the troposphere were helping to destroy the ozone and a short time later it was realized that chlorofluorocarbons (CFCs) and other compounds from human-made products (such as aerosol sprays, refrigerator/air conditioner coolants, cleaning solvents in the electronics industry and insulating plastic foam manufacturing) were being released into our air. These by-products were slowly carried into the upper atmosphere by weather patterns over a period of 1-5 years (even though CFCs are heavier than air). Once in the troposphere, these damaging chlorine atoms broke apart and were free to destroy ozone molecules (see Figure 2). In most cases, the chlorine atoms were not able to make a significant impact, but the atmospheric conditions above Antarctica were just right for the chlorine atoms to wreak havoc with the ozone layer. Ozone holes are also now appearing in the Northern hemisphere as well, usually during the winter months.
Due to this discovery, production of aerosol spray cans containing CFCs was outlawed in the U.S. in the late 1970s. But, as the years passed, it became apparent to the U.S. and other countries that more serious measures were required. In 1987, the U.S. and many other nations agreed to the Montreal Protocol, which outlined a global plan for the reduction of CFCs and other harmful compounds. Since 1988, there has been a significant decline in the production of these harmful compounds, but because the lifetime of these compounds is so long (50 – 2,000 years!), and the damage they cause so severe, it is estimated that the atmospheric ozone layer will remain damaged to some degree until 2050.
Associated Activities
- Gumdrop Ozone Depletion Model: Battling for Oxygen - Using gumdrops and toothpicks, students conduct a large-group, interactive ozone depletion model. Students explore the dynamic and competing upper atmospheric roles of the protective ozone layer, the sun's UV radiation and harmful human-made CFCs (chlorofluorocarbons).
- Metamorphosis — Stories of Change - In this literacy activity, students learn how to tell a story in order to make a complex topic (such as global warming or ozone holes) easier for a reader to grasp. Students realize that the narrative impulse underlies even scientific and technical writing, and gain a better understanding of the role of myth as a "science" of imagination that helps us to gain insight into human motivation.
Lesson Closure
Ask the students what they have learned about ozone. Review with them the process by which ozone breaks down in contact with ultraviolet radiation and is produced again when free oxygen atoms come in contact with each other and oxygen (O2) molecules. Ask them how pollution, such as CFCs, can interrupt the natural process of ozone production. (Answer: Harmful pollutants in the air, such as CFCs, can destroy ozone by converting it into oxygen molecules and atoms, and actually forcing ozone to break down more rapidly than it can rebuild.)
Ask students to think of other analogies that demonstrate the Earth's ozone layer and holes. They could be physical models, metaphors or similes.
Vocabulary/Definitions
chlorofluorocarbons: CFCs are human-made chemicals used in older aerosol sprays, older refrigerators/air conditioner coolants, cleaning solvents in the electronics industry and insulating plastic foam manufacturing that cause a thinning of the protective atmospheric ozone layer.
ozone: A form of oxygen gas (with three oxygen atoms, 03) that occupies most of the atmosphere between 10-30 miles above the Earth. When trapped at ground level, ozone is one of the most dangerous components of smog.
ozone layer: A layer of ozone gas that is part of our air or atmosphere anywhere between 10 to 30 miles above the Earth. It is important because it prevents the most harmful rays of the sun from reaching the Earth's surface.
paleobiologist: A scientist who studies the fossils of plants, animals and other organisms to learn about the Earth's ancient biology.
stratosphere: The layer of the atmosphere above the troposphere. It is 30 miles thick and contains the ozone layer.
troposphere: The layer of the atmosphere closest to the Earth. It is 10 miles high and contains all of the air in the atmosphere.
ultraviolet radiation: Harmful light rays from the sun, which can cause people to get very sick, and harm animals and plants. Classified into three forms: UV-A, UV-B and UV-C. UV-A and UV-B reach the Earth; UV-C is absorbed by the atmosphere and does not reach the Earth.
Assessment
Pre-Lesson Assessment
Discussion Question: Solicit, integrate and summarize student responses on the board. Ask the students:
- What do you know about ozone?
- What do you know about ultraviolet radiation?
Post-Introduction Assessment
Numbered Heads: Divide the class into teams of three to five students each. Have the students on each team pick numbers (or number off) so each member has a different number. Ask the students a question (give them a time frame for solving it, if desired). The members of each team should work together to answer the question. Everyone on the team must know the answer. Call a number at random. Students with that number should raise their hands to give the answer. If not all the students with that number raise their hands, allow the teams to work a little longer.
Ask the students:
- How much UV radiation does the ozone layer normally filter out? (Answer: 99%)
- True or False: The amount of ozone protecting us is decreasing. (Answer: True)
- What harmful health effects do scientists say that the ultraviolet radiation that passes through our "shield" will cause? (Answer: Increases in skin cancer, immune deficiencies and cataracts.)
- In 1987, how many U.S. citizens did the EPA estimate will get skin cancer during the next 88 years? (Answer: 40 million)
- What happens to form ozone gas? (Answer: Oxygen molecules interact with ultraviolet rays from the sun. Under normal circumstances, ozone is continuously being destroyed and regenerated by the sun's ultraviolet rays. The seasons of the year, changing winds and even sunspots affect ozone levels.)
- What are "holes" in the ozone layer? (Answer: Places where ozone concentrations are lower than under normal conditions, allowing more ultraviolet radiation to reach the Earth's surface.)
- Name a place where ozone holes exist. (Answer: Antarctica, the northern half of the U.S. into Canada and over northern Europe.)
- What is one way to stop the destruction of ozone in our atmosphere? (Answer: Reducing human-created pollutants that contribute to its depletion.)
- What are the most common ozone-destroying pollutants? (Answer: chlorofluorocarbons or CFCs.)
- From where do these ozone-destroying pollutants primarily come? (Answer: Air conditioner coolants, refrigerator coolants, industrial and cleaning solvents, aerosol spray propellants, insulating plastic foam manufacturing.)
- True or False: Engineers are working on new technologies to help save the ozone and stop its destruction by CFCs. (Answer: True)
Lesson Summary Assessment
Demonstration: Conduct this activity as either a class demonstration, or if you have enough supplies, in groups of 2-4 students each. Gauge students' depth of knowledge with the concluding discussion questions. You need: waxed paper, a globe and a flashlight. Procedure:
- Hold a piece of waxed paper a few inches above the globe.
- Shine a light on the waxed paper from above. Observe how the light shines on the globe.
- Tear a small hole in the waxed paper and again shine the light on it from above. Observe how the light shines on the globe.
- Ask the students: How did the amount of light on the globe change after the hole was put in the paper? (Answer: It increased.) And, how is the waxed paper similar to the Earth's ozone layer? (Answer: It prevents much of the sun's rays from reaching the Earth.)
Save the Ozone!: "Even if all CFC use was halted today, the CFCs already released will continue to break down in the stratosphere and destroy ozone for decades." Read this sentence aloud to the students and hold a short discussion about what it means. Ask the students what they think engineers can do to help save the ozone. (Possible answers: Design technology to clean up pollutants or prevent future pollutants from entering the atmosphere, design technologies to manufacture products in non-polluting ways, persuade governments and corporations to agree to stop producing CFCs in their own countries.) Have the students create an informative flyer on ozone depletion. They should include the natural process of ozone, how it is affected by pollution, and what this means for their future. Display these flyers in the school hallway or common area.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Using gumdrops and toothpicks, students conduct a large-group, interactive ozone depletion model. Students explore the dynamic and competing upper atmospheric roles of the protective ozone layer, the sun's UV radiation and harmful human-made CFCs (chlorofluorocarbons).

Students are introduced to the concepts of air pollution, air quality, and climate change. The three lesson parts (including the associated activities) focus on the prerequisites for understanding air pollution. First, students use M&M® candies to create pie graphs that express their understanding o...

After seeing ultraviolet-sensitive beads change color and learning how they work, students learn about skin anatomy and the effects of ultraviolet radiation on human skin, pollution's damaging effect on the ozone layer that can lead to increases in skin cancer, the UV index, types of skin cancer, AB...

Through an overview of some of the environmental challenges facing the growing and evolving country of China today, students learn about the effects of indoor and outdoor air pollution that China is struggling to curb with the help of engineers and scientists.
References
Glencoe Science – An Introduction to the Life, Earth and Physical Sciences. OH: Glencoe/McGraw-Hill, 1999.
High Level Ozone. Updated October 15, 2004. School of Chemistry, University of Bristol, UK. www.chm.bris.ac.uk. Accessed November 10, 2004.
Kerrod, Robin. Let's Investigate Science – The Environment. New York: Marshall Cavendish Corporation, 1994.
Man-Made Chemicals, CFCs. Iowa State University. www.ge-at.iastate.edu. Accessed November 10, 2004.
Markle, Sandra. The Kids' Earth Handbook. Atheneum, NY: Maxwell Macmillan International, 1991.
Ozone Depletion Index. Updated June 15, 2004. Internet FAQ Archives, FAQS.ORG. www.faqs.org/faqs. Accessed November 10, 2004.
Stratospheric Ozone Information for Children. Updated September 20, 2002. Kid Zone, The Green Lane, Environment Canada. Formerly found at: www.ec.gc.ca/ozone. Accessed November 10, 2004.
Stratospheric Ozone – The Ozone Layer – What's going on up there? Updated September 20, 2002. Kid Zone, The Green Lane, Environment Canada. Formerly found at: www.ec.gc.ca/ozone. Accessed November 10, 2004.
Copyright
© 2004 by Regents of the University of Colorado.Contributors
Amy Kolenbrander; Janet Yowell; Natalie Mach; Malinda Schaefer Zarske; Denise CarlsonSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation GK-12 grant no. 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: September 3, 2020






User Comments & Tips