Quick Look
Summary
How can an understanding of pH—a logarithmic scale used to identify the acidity or basicity of a water-based solution—be used to design and create a color-changing paint? This activity provides students the opportunity to extract dyes from natural products and test dyes for acids or bases as teams develop a prototype “paint” that is eventually applied to help with a wall redesign at a local children’s hospital. Students learn about how dyes are extracted from organic material and use the engineering design process to test dyes using a variety of indicators to achieve the right color for their prototype. Students iterate on their dyes and use ratios and proportions to calculate the amount of dye needed to successfully complete their painting project.Engineering Connection
Knowledge and understanding of pH is crucial in many aspects of engineering. In pH testing, engineers can understand acids and bases and their impact on many practices that are used in industry, research and development, and in consumer products. Effective use of pH testing has a measurable impact across many engineering fields, particularly in biological and chemical engineering and is an essential component in research and engineering design.
Learning Objectives
After this activity, students should be able to:
- Safely extract dye from organic material using water.
- Accurately test pH of various substances.
- Determine if a substance is acidic or basic.
- Discuss how substances change color in the presence of application of indicators.
- Determine proportional relationships.
- Use proportional relationships to determine scaled quantities.
- Create a multimedia component and include it in a presentation that clarifies findings and emphasizes salient points.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-ETS1-2. Evaluate competing design solutions using a systematic process to determine how well they meet the criteria and constraints of the problem. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Evaluate competing design solutions based on jointly developed and agreed-upon design criteria. Alignment agreement: Collect data to produce data to serve as the basis for evidence to answer scientific questions or test design solutions under a range of conditions.Alignment agreement: | There are systematic processes for evaluating solutions with respect to how well they meet the criteria and constraints of a problem. Alignment agreement: | |
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-ETS1-3. Analyze data from tests to determine similarities and differences among several design solutions to identify the best characteristics of each that can be combined into a new solution to better meet the criteria for success. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Analyze and interpret data to determine similarities and differences in findings. Alignment agreement: | There are systematic processes for evaluating solutions with respect to how well they meet the criteria and constraints of a problem. Alignment agreement: Sometimes parts of different solutions can be combined to create a solution that is better than any of its predecessors.Alignment agreement: Although one design may not perform the best across all tests, identifying the characteristics of the design that performed the best in each test can provide useful information for the redesign process—that is, some of the characteristics may be incorporated into the new design.Alignment agreement: | |
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-ETS1-4. Develop a model to generate data for iterative testing and modification of a proposed object, tool, or process such that an optimal design can be achieved. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to generate data to test ideas about designed systems, including those representing inputs and outputs. Alignment agreement: | Models of all kinds are important for testing solutions. Alignment agreement: The iterative process of testing the most promising solutions and modifying what is proposed on the basis of the test results leads to greater refinement and ultimately to an optimal solution.Alignment agreement: | Models can be used to represent systems and their interactions. Alignment agreement: |
Common Core State Standards - Math
-
Represent proportional relationships by equations.
(Grade
7)
More Details
Do you agree with this alignment?
-
Recognize and represent proportional relationships between quantities.
(Grade
7)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the attributes of design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of engineering design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of the role of troubleshooting, research and development, invention and innovation, and experimentation in problem solving.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop abilities to apply the design process.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Ohio - English
-
Include multimedia components and visual displays in presentations to clarify claims and findings and emphasize salient points.
(Grade
7)
More Details
Do you agree with this alignment?
-
Write informative/explanatory texts to examine a topic and convey ideas, concepts, and information through the selection, organization, and analysis of relevant content.
(Grade
7)
More Details
Do you agree with this alignment?
Ohio - Math
-
Analyze proportional relationships and use them to solve real-world and mathematical problems.
(Grade
7)
More Details
Do you agree with this alignment?
-
Represent proportional relationships by equations.
(Grade
7)
More Details
Do you agree with this alignment?
Materials List
Part 1 (Day 1)
Each student needs:
Teacher Demonstration needs:
- two 250 mL beakers
- hot plate
- tap water
- pH indicator litmus paper, or a pH probe (if accessible) with buffers
- dish towel
- strainer
- rubber mallet, food chopper, or mortar and pestle
- scale, electronic or triple beam balance
- vegetables, fruits, spices and beans, or flowers for demonstration (vegetables - beets, onion peels, red cabbage, spinach; fruits - berries, cherries; spices and beans - turmeric, coffee, tea; flowers - marigolds, dandelions, zinnia;) as an example, one pint of strawberries or blueberries for color extraction works well
Part 2 (Day 2-4)
Each student needs:
- Organic Chemist Recording Guide for Applying Natural Dyes
- Organic Chemist Recording Guide for Extracting Natural Dyes
- Additional Data Chart (optional)
- Day 2 Exit Slip
- Technician’s Plan Sheet
Each group needs:
- three different types of organic materials from Day 1 (students can select these)
- three 250 mL beakers
- strainer
- tap water
- hot plate
- rubber mallet, mortar and pestle, or food chopper
- safety goggles
- three 1-gallon Ziploc bags
- dish towels
- three stir rods
- paint brushes
- three to six pieces of copy paper or watercolor paper per dye. Suggest strips 0.00581m2 (1/16 ft2) in size. (tested with: Strathmore Paint Pad, 100 Series https://www.strathmoreartist.com/kids/strathmore-100-seriesyouth-paint-pad.html)
- 100 mL beakers or test tubes of the three test solutions: white vinegar 5%, lemon juice, red cabbage water, baking soda solution
- several droppers (plastic, 1 mL)
- hair dryer or fan
- pH paper (provide lots of pieces for experimentation!) or a pH probe with buffers
- scale, electronic balance or triple beam balance
- three Organic Chemist Recording Guide for Applying Dyes
Part 3 (Day 5-6)
Each student needs:
- Organic Chemistry Lab Sheet
- Proposal Guidelines for Verbal Presentations (for verbal presentations) or Proposal Guidelines for Written Presentations (for written)
- Organic Chemist Recording Guide for Extracting Dyes
- Activity Post-Quiz
Each group needs:
- computers with presentation software (verbal), google doc (written)
- Proposal Checklist for Verbal Presentations or Proposal Checklist for Written Presentations
To share with the entire class (if using verbal presentations):
- computer with projector
- easel
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/uod-2271-color-change-paint-ph-engineering-design-challenge] to print or download.Pre-Req Knowledge
Students should understand the characteristics of acids and bases and how to measure pH. They should be able to write a ratio and unit rate, write and solve a proportion, write the equation that represents the proportional relationship, and graph a proportional relationship.
Introduction/Motivation
A group of board members at local children’s hospital are investigating ways to better serve their young patients through better design aesthetics. Currently, the walls of the intake area at the hospital are a bland tan color and do not account for patient preferences. This examination room is often the first stop for young patients entering the hospital, and they are often in this room for hours while they await exams, tests, and decisions about possible admission. This can be a scary time for young patients and the hospital would like to provide patients with the novel ability to change the color of this room if they do not feel relaxed or comfortable. This change must be quick, easy, and efficient. Your new paint might be the perfect choice, but you will need to know more details before you can propose this to the hospital board! This exam room only has one wall that can be painted as the other walls are reserved for storing examination equipment.
The board has requested your engineering team’s assistance in designing and developing this paint prototype. Their ultimate hope is to provide their patients a little control in what typically is an uncontrollable situation.
Your engineering team will need to explore, create, and test three different natural dyes to determine if it is possible to create this type of paint. The board has also requested a brief multimedia presentation and demonstration of the proposed paint prototype that you and your engineering team will develop.
How can you use your knowledge of pH to design and create a color-changing paint?
Procedure
Background
What is pH?
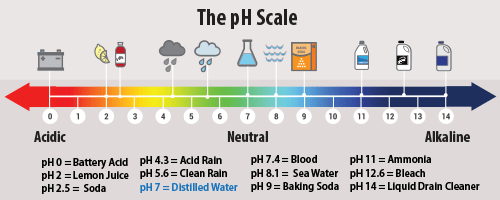
The potential of hydrogen (pH) is the measurement of the concentration of hydrogen ions (H+) in a solution. Scientists use pH level to classify a solution as acidic, basic (sometimes referred to as alkaline), or neutral. If the hydrogen ion (H+) concentration is high (pH below 7), the solution is classified as an acid. If the hydrogen ion (H+) concentration is low (pH above 7), the solution is a base. When a solution, such as water, has an equal amount of acidic and alkaline molecules, it is classified as neutral with a pH of 7.
The pH scale is logarithmic (with a 10-fold difference between successive whole numbers), and goes from 0 to 14, with 7.0 being neutral. Some extreme substances can score lower than 0 or greater than 14, but most fall within the scale. There are several methods used to determine a solution’s pH level. Liquid indicators are weak acids and exist as liquid dyes and in dye form. These can be added directly to the solution, resulting in a change of color. Paper indicators, such as litmus paper, are dipped into the solution and then removed and compared to a color key for classification. Additionally, pH indicators can be found in nature and their presence in plants and flowers can indicate the pH of the soil in which they grow.
What makes it possible for red cabbage and other organic materials to be indicators?
Anthocyanins are water-soluble pigments found mostly in flower and fruit cells of plants, but are also in leaves, stems, and roots. Anthocyanins may appear as red, blue or purple in color, depending on their pH. When their pH is changed, the plant’s color also changes as a result: pink in acidic solutions, purple in neutral, and greenish-yellow in base solutions. Solutions on the extreme end of the base scale may result in a colorless change.
Anthocyanins consist of many carbon rings with hydrogens attached. This molecule can take two forms, in one the hydrogen is attached to the exterior, the other in the interior. When exposed to an acid, anthocyanins grab a hydrogen atom and change to a red color. When exposed to a base, there are no excess of hydrogen atoms, the molecule appears blue or green.
Foods with high anthocyanin content are blueberries, cranberries, raspberries, blackberries, cherries, eggplant peels, Concord grapes, red cabbages, red-fleshed peaches and violets. Foods with less abundant anthocyanin include bananas, asparagus, peas, fennel, pears and potatoes.
Before the Activity
- Prior to the start of the activity, the teacher should instruct students to brainstorm and gather materials to bring for use in their dye extracts such as different varieties of vegetables: beets, onion peels, red cabbage, spinach; fruits: berries, cherries; spices and beans: turmeric, coffee, tea; flowers: marigolds, dandelions, zinnia. Students should not bring in wild or unknown plants: (See “Safety Issues” below.)
- Purchase plant material for dye extraction. Students may also choose to bring plants in from home.
- Organize materials and make one copy of the Activity Pre/Post-Quiz and Day 2 Exit Slip per student and one copy of the following for each group: Engineering Design Challenge Rubric, Organic Chemistry Lab Sheet, Organic Chemist Recording Guide for Extracting Natural Dyes, Organic Chemist Recording Guide for Applying Natural Dyes, Technician’s Plan Sheet, Proposal Guidelines for Verbal Presentations, Proposal Guidelines for Written Presentations (if using written presentations), Proposal Checklist for Verbal Presentations (for verbal), Proposal Checklist for Written Presentations (for written).
- Review the Technical Brief for background information on the dye extraction process using water.
With the Students
Day 1:
- Place students in groups of three to four.
- Administer the Activity Pre/Post-Quiz.
- Introduce the engineering design challenge and Engineering Design Challenge Rubric.
- Demonstrate the dye extraction process and pH testing for the class. You may follow the suggested extraction process using water below. This is not the only means of extracting dye, but this water extraction method was tested and found to be successful.

- Instruct students to prepare their organic material by exposing as much surface area as possible. Place material in gallon Ziploc bag wrap with dish towel or paper towel prior to smashing with hammer/mallet or by using a small food chopper.
- To prepare the dye, you will need to mix water and organic material using a volume ratio of 2:1 tap water to organic material.
- Mix the water and organic material in a beaker and bring the mixture to a boil. Simmer the mixture on a hot plate for a minimum of 30 minutes and up to one hour. Use glass covers on beakers to prevent splashing. To save time during demonstration, prepare one beaker per demonstration ahead of time so you can demonstrate the boiling and simmering phases.
- Using a glove or oven mitt, strain the dye into a beaker. Be careful, the liquid will be hot!
- Demonstrate the process and procedure you will use for pouring the dye into the storage bag and label each sample by name. Store each dye in a dark, cool place for later use.
- Demonstrate /review the procedure you plan on students using for cabbage water, pH paper and/or probe to test pH to identify if dye is acidic, basic or neutral.
- Conclude your demonstration with a Q & A for students. This allows students to come up with probing questions or to ask questions that may help clarify the procedure.
Day 2- 4:
- Remind students that the Engineering Design Process is cyclical and they will be extracting, applying, and changing colors for several dyes. This process will enable their group to present their client with the best option. Suggest that students first extract a minimum of three dyes, followed by dye testing, application, and evaluation.
- Extraction:
- With groups, students begin extracting dyes from their selected materials using the same process demonstrated by the teacher on Day 1. Students should complete the Organic Chemist Recording Guide for Extracting Natural Dyes and the Organic Chemist Recording Guide for Applying Dyes during the extraction process.
- The Additional Data Chart can assist students in collecting the data they will need to complete the homework assignment on Day 4.
- Testing and application of dyes to paper:
- As students compare dyes, they should take pH testing samples and record these data and observations. The teacher may want to have additional pre-made dyes available for groups to use if time for extractions runs short or if student-selected dye material does not provide adequate amounts of dyes.
- Apply the dyes to paper measuring 2.5 cm2 (approximately 1 in x 1 in) using brushes; three to six pieces per material will allow students to apply multiple indicators per dye. Allow the first coat to dry and use a fan or hair dryer to quicken the drying process. Apply the second coat and allow it to dry. Make sure to label each paper with the type of dye used.
- Indicator applications
- Students should select a solution to the test and record color change. Options include: 5% white vinegar, lemon juice, red cabbage water, and a solution of baking soda and water.
- Using either a brush or a dropper, students will then apply their solutions to dyed paper and record their observations.
- Have students evaluate the results and record observations.
- Remind students to take pictures of the dyes and color changes for later use in a presentation and to label each paper with dye type and indicator they used.
Note: Administer the Exit Slip at the end of Day 2.
Note: Ensure students complete the Organic Chemistry Lab Sheets and both the Organic Chemist Recording Guide for Extracting Natural Dyes and Organic Chemist Recording Guide for Applying Natural Dyes by the end of Day 4. Distribute the Technician’s Plan Sheet as homework.
Day 5-6:
- Students should finalize their dye selection for presentation. Circulate the room and assist student groups in making their decision by asking for student rationale of why they chose a particular dye over another based on the data.
- Use this time during student redesign to check each individual’s Technician’s Plan Sheet, and assist as needed.
- Distribute and review Proposal Guidelines for Verbal Presentations with student groups. These guidelines help students create their multimedia presentations. The Proposal Guidelines for Written Presentations provides tips on how to present using a Google Doc rather than a PowerPoint presentation.
- Instruct students to being writing and creating presentations. Circulating throughout the groups, scanning presentation drafts for completeness, accuracy and clarity. Advise students to refer to the rubric and lab sheets as needed.
Day 6:
- To finalize the project, student groups should present their proposals either in verbal or written format (depending on which format the teacher chooses.) Use the Proposal Checklist for Verbal Presentations during the verbal presentations, or the Proposal Checklist for Written Presentations for written assignments.
- Re-administer the Activity Pre/Post-Quiz.
Vocabulary/Definitions
acid : Any class of compounds that form hydrogen ions when dissolved in water, and whose aqueous solutions react with bases and certain metals to form salts. Acids turn blue litmus paper red and have a pH level below 7.
alkali : A base that dissolves in water, or a solution of a soluble base has a pH greater than 7.0. (Not be confused with basicity which is an absolute measurement on the pH scale.)
anthocyanin : A water-soluble pigment that, depending on the compound’s pH, may appear red, purple, or blue.
base : A substance that can accept a hydrogen ion from another substance and considered the “chemical opposite” of an acid. Bases turn red litmus paper blue and have a pH level above 7.
indicator: Chemicals that show whether the given solution is acidic or basic by a sudden change of color.
pH scale: In chemistry, a logarithmic scale that determines whether a solution is acidic or basic; 7 (such as pure water) is neutral, while 6 to 0 denotes an acid and 8-13 denotes a base.
proportion: A mathematical comparison between equivalent ratios.
prototype: A 3d model.
ratio: A mathematical comparison between two quantities.
Assessment
Pre-Activity Assessment
Pre-Quiz: Students complete the Activity Pre/Post-Quiz independently prior to teacher demonstration to assess their knowledge of objectives.
Activity Embedded Assessment
Exit Slip: Students complete the Day 2 Exit Slip independently at the end of Day 2.
Technician’s Plan Sheet: Students complete the Technician’s Plan Sheet independently or with group (teacher discretion) at the end of Day 4. There are three tiers of homework to assist in matching student math level to homework.
Hospital Board Proposal: Use the following checklists to assess student presentations: Proposal Checklist for Verbal Presentations or Proposal Checklist for Written Presentations.
EDC Rubric: Use the Engineering Design Challenge Rubric to assist in presentation creation and assessment.
Post-Activity Assessment
Post-Quiz: Students re-take the Activity Pre/Post-Quiz independently following completion of presentations.
Safety Issues
- Use eye protection (goggles or safety glasses) during the dye creation/use phase of activity.
- Use caution near the hot plate/dye.
- Use glass covers on beakers during dye extraction.
- Check-in with the school nurse to see what kind of organic materials may pose an allergy risk, if any.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are introduced to acids and bases, and the environmental problem of acid rain. Students also conduct a simple experiment to model and discuss the harmful effects of acid rain on our living and non-living environment.

Students learn the basics of acid/base chemistry in a fun, interactive way by studying instances of acid/base chemistry found in popular films such as Harry Potter and the Prisoner of Azkaban and National Treasure. Students learn what acids, bases and indicators are and how they can be used, includi...

Students learn about the basic properties of light and how light interacts with objects. They are introduced to the additive and subtractive color systems, and the phenomena of refraction. Students further explore the differences between the additive and subtractive color systems via predictions, ob...
Copyright
© 2018 by Regents of the University of Colorado; original © 2016 University of Dayton, Central State University, and Wright State University.Contributors
Joseph Duncan; Linda Gillum; Miyong Hughes; Benjamin McCombs; Carly MonfortSupporting Program
Collaborative RET Program, University of Dayton, Central State University, and Wright State UniversityAcknowledgements
This material is based upon work supported by the National Science Foundation under grant no. EEC 1405869—a collaborative Research Experience for Teachers Program titled, “Inspiring Next Generation High-Skilled Workforce in Advanced Manufacturing and Materials,” at the University of Dayton, Central State University and Wright State University in Ohio. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Last modified: February 28, 2021






User Comments & Tips