
Summary
Students are challenged to efficiently extract a model rare earth element, terbium, which is essential for electronics but typically refined using harmful acid-washing methods. Students first learn about water and solution properties such as hydrophobic/hydrophilic interactions, solubility, and the effects of temperature and agitation. They then apply this knowledge to a simulated extraction challenge: separating black pepper (terbium) from a solution of water (acid), salt (calcium), and sugar (iron). Finally, competing student groups must determine the fastest, most cost-effective combination of variables (temperature, agitation, etc.) to dissolve the salt and sugar, leaving only the "purified" pepper, thereby modeling the innovative and fiscally responsible problem-solving used by environmental engineers to safely extract rare earth elements.Engineering Connection
Environmental engineers are challenged to create eco-friendly and cost-effective solutions for metal ore mining and refining amid climate change. To tackle large-scale problems, engineers work on small-scale models in labs that can be scaled up for industry. They plan based on prior research, collect and analyze data through iterations, collaborate with others, and continuously test and improve solutions. Students will follow the same engineering process using affordable and safe alternatives to acid and metal ores on a small scale.
Learning Objectives
After this activity, students should be able to:
- Describe aqueous solutions in terms of solute and solvent, and concentration.
- Describe up to four ways to speed up the dissolution process of a solute into a solvent.
- Investigate and model how the temperature of solvent, surface area of solute, and agitation of solution affect the rate of dissolution of solid solutes in aqueous solutions.
- Evaluate competing design solutions using a systematic process to determine how well they meet the criteria and constraints of the problem.
Educational Standards
Each Teach Engineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each Teach Engineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in Teach Engineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-ETS1-2. Evaluate competing design solutions using a systematic process to determine how well they meet the criteria and constraints of the problem. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Evaluate competing design solutions based on jointly developed and agreed-upon design criteria. Alignment agreement: | There are systematic processes for evaluating solutions with respect to how well they meet the criteria and constraints of a problem. Alignment agreement: | |
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS1-3. Gather and make sense of information to describe that synthetic materials come from natural resources and impact society. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Gather, read, and synthesize information from multiple appropriate sources and assess the credibility, accuracy, and possible bias of each publication and methods used, and describe how they are supported or not supported by evidence. Alignment agreement: | Each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. Alignment agreement: Substances react chemically in characteristic ways. In a chemical process, the atoms that make up the original substances are regrouped into different molecules, and these new substances have different properties from those of the reactants.Alignment agreement: | Structures can be designed to serve particular functions by taking into account properties of different materials, and how materials can be shaped and used. Alignment agreement: Engineering advances have led to important discoveries in virtually every field of science, and scientific discoveries have led to the development of entire industries and engineered systems.Alignment agreement: The uses of technologies and any limitations on their use are driven by individual or societal needs, desires, and values; by the findings of scientific research; and by differences in such factors as climate, natural resources, and economic conditions. Thus technology use varies from region to region and over time.Alignment agreement: |
State Standards
Texas - Science
-
describe aqueous solutions in terms of solute and solvent, concentration, and dilution; and
(Grade
7)
More Details
Do you agree with this alignment?
-
investigate and model how temperature, surface area, and agitation affect the rate of dissolution of solid solutes in aqueous solutions.
(Grade
7)
More Details
Do you agree with this alignment?
Materials List
For the class to share:
- 1 lb. granulated salt
- 1 lb. granulated sugar
- 1 lb. granulated pepper
- 50 coffee filters
- pack of Himalayan chunky salt (at least 5 g needed per class period)
- pack of large/coarse sugar crystals (at least 5 g needed per class period)
- pack of large peppercorn kernels (at least 5 g needed per class period)
- 50 Styrofoam cups
- 100 clear small cups/disposable cups
- 24 coffee stirrers
- 24 straws
- (optional) printed copies of periodic tables for students to keep
- scales (4 or more is ideal, but 2 will work)
- paper towels (at least 1 pack per class period)
- printed copies of periodic tables for students to reference
- chart paper (optional if you have lots of whiteboard space)
- markers (optional if you have lots of whiteboard space)
- electric kettle
- ice/cooler/refrigerator access
- 24 spoons (to be reused)
- 9 large beakers (500 – 1000 mL) or large glass cups/jars
- 18 medium beakers (250 – 500 mL) or smaller cups/jars
- 20 clear small plastic cups (to be reused for other class periods)
- 8 graduated cylinders (10 mL) (if on a tight budget, 4 is fine)
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/uot-3002-mines-mobiles-aqueous-solutions-activity] to print or download.Pre-Req Knowledge
Students should:
- Be familiar with lab safety (e.g., heat, splashing chemical solutions, sharing supplies).
- Have a rudimentary understanding of mines and mining.
- Understand how items dissolve in our daily lives (e.g., dissolving a drink flavor packet into a bottle of water).
- Understand that acids are corrosive to human skin.
Introduction/Motivation
Who has a phone or computer with them or at home? Have you ever looked inside to see what is there? What are phones made of? What metals do they use? Where do we get these metals? (Wait for responses and list some on the board.)
Phones are often made of many metals, but we particularly care about lanthanides (rare earth elements) for electronic and touch displays. Metals come from the Earth’s crust, and we get them through mining. Mining involves two main steps: digging up the metals, and then purifying them. Phones and computers mostly use special metals called rare earth elements (REEs). Even though they are called "rare," they are not really that rare. They are hard to find in a “pure” form; they are often mixed with other metals such as iron and calcium. What are some other metals we mine for? (Have students do a partner share and then have 2-3 people share answers with whole class.)
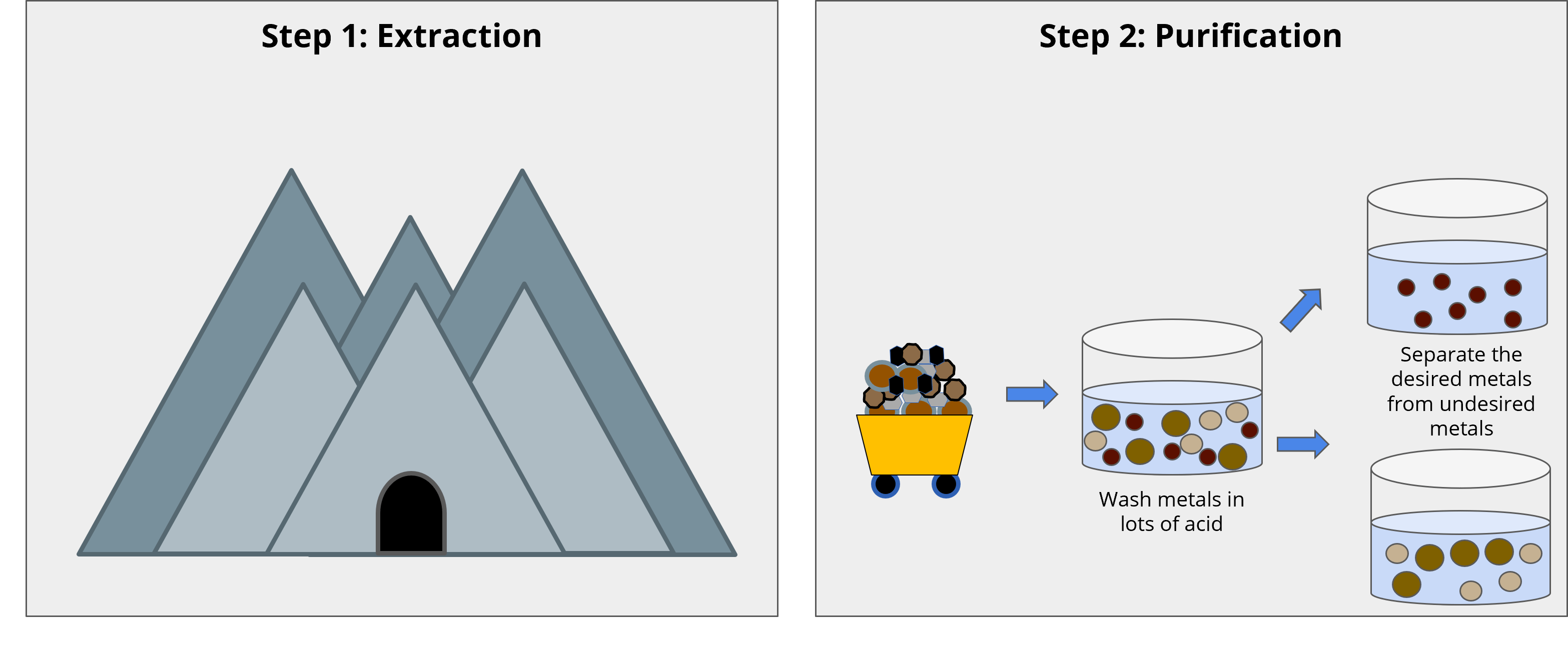
When miners dig up these metals, they get a mixture of REEs, calcium, and iron. They need to separate them to isolate the REEs, which is tricky. Usually, they use acid to wash away the unwanted metals. However, this can harm the local environment and be dangerous for workers. The acid can leak into nearby water sources, hurting the surrounding plants, animals, and people. Metal purification also happens in facilities that use a lot of energy and fossil fuels, which contributes to more air pollution. Have you ever seen pollution? How did it affect the surrounding ecosystem? (Allow students to share with each other.)
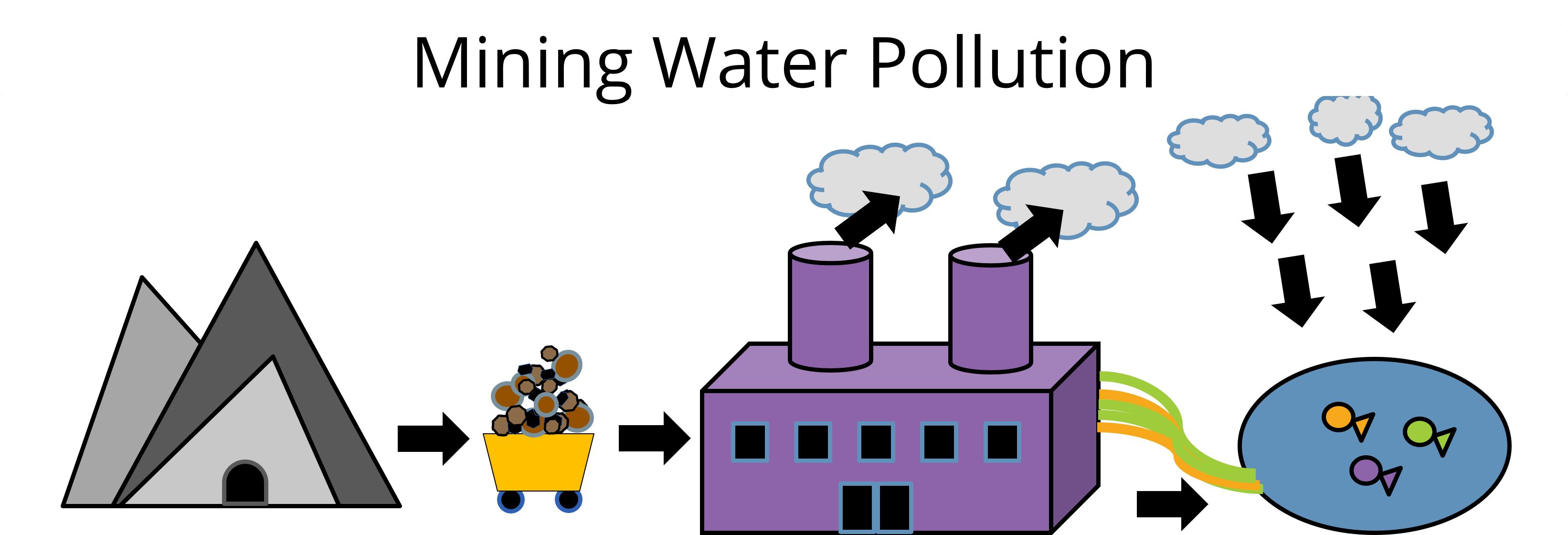
That’s why we need better, safer ways to separate these metals. Environmental engineers are working on new methods that are safer and more eco-friendly. They start with small models in the lab and then scale up their ideas for real-world use. They do a lot of planning, testing, and improving to find the best ways to mine and purify metals.
In class, we will use a safe experiment to understand this process. We will use water, salt, sugar, and pepper. The water will act like acid, the pepper will be the REEs, the salt will be calcium, and the sugar will be iron. Can you find calcium (Ca) and iron (Fe) on the periodic table? (Show a periodic table slide and ask one student to come up and point to calcium and iron.)
Now, let’s brainstorm. What do you already know about salt, sugar, pepper, and water? What happens when we mix them together? (Have students do a partner share, then have 2-3 people share answers with the whole class. Hand out the “pepper extraction model” and have students complete in groups for about 5 mins. Then, call on students to share answers. You should have your own copy on the document camera/drawing it on an anchor chart. This will be referred to often).
Procedure
Background
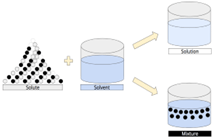
An aqueous solution is one in which water is the solvent, the liquid that dissolves solutes. Solutes are liquids or solids that can be dissolved in a solvent. Dissolving means that a solute goes into the solution and combines with the solvent. A mixture, on the other hand, consists of items in the same area that do not mix with each other and remain independent. To encourage the dissolution process, you can increase the temperature of the solution, decrease the size of the solute, or dilute the solution to decrease its concentration.
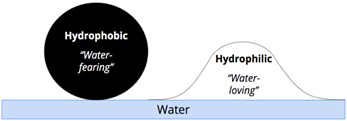
Substances can be either hydrophobic or hydrophilic. Hydrophobic substances, meaning "water-fearing," do not dissolve well in water; common examples include oil and water or black pepper. Hydrophilic substances, meaning "water-loving," dissolve well in water; salt is a common example. The dissolution point is reached when no more solute can dissolve in the solution, even if it is hydrophilic, due to the high concentration of the solute.
Research Jigsaw Structure
Rather than looking at articles/videos about aqueous solutions, students will spend the research step of the engineering process by investigating how they can expedite the dissolving process on their own. Students are divided into groups of four and assigned to one of four stations:
- Station 1: Investigate the effects of increasing solute concentration on solutions using salt, sugar, and pepper.
- Station 2: Study how the differing surface area of solutes (table salt vs. Himalayan sea salt chunks, granulated sugar vs. sugar crystals, and table pepper vs. peppercorns) affects dissolution rates.
- Station 3: Analyze how solvent temperature (ice cold, room temperature, hot water) impacts the dissolution rate of table salt, sugar, and pepper.
- Station 4: Examine the effects of agitation (no agitation, 30 seconds, and 60 seconds of gentle agitation) on the dissolving rates of table salt, sugar, and pepper.
This activity goes through an entire engineering cycle over five 45-minute sessions. Preparing all handouts and wet-lab supplies will be essential before each class period begins.
Before the Activity
- Print all handouts prior to activity.
- Pre-assemble all lab research stations for Day 2 per the Research Station Table Directions and Research Station Preparation handout.
- Pre-assemble all “shipments” of metals for both Day 3 and Day 4 per the AB Shipments Materials Prep.
- Have spoons, straws, hot water, graduated cylinders, cups, a. all readily available for student use on Day 3 and Day 4.
- Discuss lab cleanup procedures/write them on the board or chart paper and refer to them throughout the activity.
- Be sure to test the experiment yourself at least once to mitigate any speedbumps that may arise for your classroom circumstances (e.g., if the microwave takes too long to heat the water, is there another tool you can use?)
Day 1: Ask (45 mins)
- Put students into groups of four.
- Do Now Activity: Give groups three minutes to complete the warm-up questions. (Slide 3 of Day 1 Slides)
- Describe the “Ask” step of the engineering design process. (Slide 4)
- Introduce students to the problem with Slides 5-10. See speaker notes on each slide for further information.
- Pose the questions on Slide 5.
- Give students two minutes to brainstorm ideas for the questions.
- As a class, discuss student answers and list their responses on the board.
- Show them the metals commonly needed for phones and computers. (Slides 6-7)
- Explain that these metals come from the earth's crust through mining, which involves two main steps: extraction (digging out the metals) and purification (using an acid washing process that causes pollution). (Slide 8)
- Discuss mining pollution. (Slide 9)
- Discuss how environmental engineers are developing safer mining techniques. (Slide 10)
- Present students with the Design Challenge: Students will assume the roles of environmental engineers tasked with designing an eco- and budget-friendly alternative to acid washing.
- Provide more background information for the design challenge with Slides 11-12.
- Show a periodic table and ask students to find calcium, iron, and terbium. (Slide 11)
- Read aloud the speaker notes on Slides 11-12.
- Show Slides 13-14 and survey for student knowledge about how salt, sugar, and pepper behave in water.
- Optional: Give students 5-10 minutes to complete the Pre Test worksheet. (Slide 15)
- Specifically define the problem:
- Pass out the Pepper Extraction Model/Note Catcher to each student.
- Have students define the problem: separating a rare earth element (pepper) from other substances (sugar and salt) in a solution. (Slide 16)
- Give teams 5 minutes to answer the questions in the Pepper Extraction Model/Note Catcher with each other.
- Walk around the room to help them answer these questions and clarify any confusion.
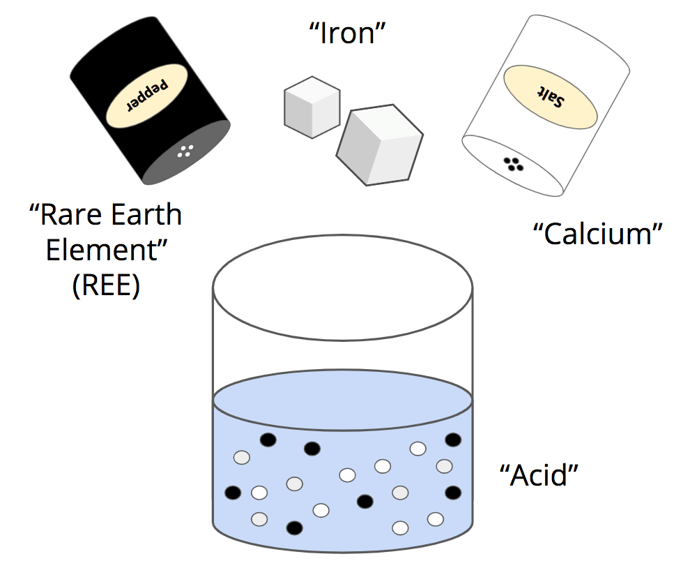
- Identify the criteria and constraints:
- Optional: Give students 3 minutes to brainstorm ideal criteria and constraints. This step may be hard, but the more ideas they produce as a class the better. (Slide 17)
- As a class, spend 3 minutes brainstorming. Be sure to clearly define what criteria and constraints are. (Slide 18)
- Give groups time to use the Pepper Extraction Model/Note Catcher to map out the problem they are trying to solve and key vocabulary for the activity.
Day 2: Research (45 mins)
- Describe the “Research” step of the engineering design process. (Slide 3 of the Day 2 Slides)
- Explain the Jigsaw Research Activity (Slides 4-5)
- Have students form groups of 4 (or 5 if needed).
- Have students number themselves 1, 2, 3 or 4.
- Direct students to their assigned stations.
- Review safety procedures for each station. (Slide 6)
- Give each student an Aqueous Solutions Research Data Sheet.
- Have students review their specific station instructions in Research Station Table Directions.
- Display Slide 7 of the Day 2 Slides with instructions for the duration of the research time so students can guide themselves.
- Give students 15 minutes to work with their station to collect and discuss data.
- Direct students to quickly clean up their station areas.
- Hand out the Jigsaw Key Patterns worksheet, one per student. (Slide 8)
- Have students return to their original groups of four and take turns sharing their findings. (Note: Although they are acting as a team, each member of the group should be taking notes in their Jigsaw Key Patterns handout to ensure learning and to mitigate lost data from another member.)
- As students share their Jigsaw findings, circulate and guide them to notice patterns and clarify misunderstandings.
- Bring the class back together and do a whole class debrief: summarize research, connect to mining challenges and solubility, introduce terms (solute, solvent, solution, hydrophobic, hydrophilic), and update pepper extraction models. (Slides 9–12)
- Show the salt and sugar Solubility Graph and discuss whether results match student data, including why sugar and salt behave differently. (Slides 13-14)
Day 3: Imagine and Create (45 mins)
- Describe the “Imagine, Plan and Create” steps of the engineering design process. (Slide 3 of the Day 3 Slides)
- Hand out the Imagine Ideal Scenarios to each student.
- Recap patterns that students saw in their research during the previous class period. (Slide 4)
- Have students practice different solubility scenarios in the Imagine Ideal Scenarios sheet. (Slides 5-7)
- Provide the following scenario to the class: Each group will receive a ‘random’ 10 grams shipment containing 3 grams of pepper. The remaining 7 grams vary in salt and sugar, with some shipments salt-heavy and others sugar-heavy. Students must write a unique extraction plan based on their research. (Slide 8)
- Give each student a Pepper Purification Plan handout.
- Give the students 10 minutes to plan their method. (Slide 9)
- With an ideal method in mind, give students 10-15 minutes to try their ideas on their designated assortment of spices. (Slide 10)
- Students will try different combinations of stirring and hot or cold water to dissolve their “calcium” and “iron.”
- Students must get all of their salt and sugar to dissolve, leaving behind pepper floating in an aqueous solution in their cup.

- Before the end of the class, have students let their filtered pepper dry overnight. It is advised that the students filter their solutions into a disposable cup or glass beaker. The glass beaker is easy to clean and the disposable cup can be thrown away easily. Note: The next day, they will come back and take the mass of their pepper to see if they successfully extracted 3 grams. (Slide 12)

Day 4: Test and Improve (45 mins)
- Describe the “Test and Improve” steps of the engineering design process. (Slide 3 of the Day 4 Slides)
- Have students weigh the pepper they extracted previously and record the mass on their Pepper Extraction Plan handout. Note: Each group, no matter which shipment they got, should have extracted up to 3 grams of pepper.
- Have students reflect on their methods for improvement. (Slides 5-6)
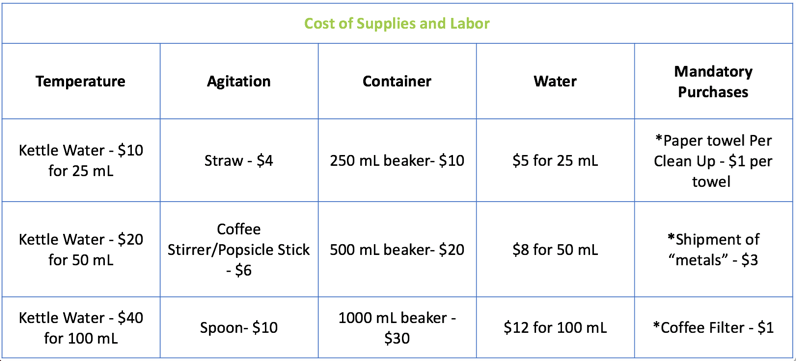
- Introduce the Engineering Competition. (Slide 7)
- Groups are given a list of costs for labor and materials and they must now plan to extract pepper from another allotment of their same shipment at the cheapest cost.
- All groups are now competing against each other to “purify” their pepper the cheapest, as If they were startup engineering firms competing for a job.
- Because students have different amounts of sugar and salt, they will likely be “purchasing” different combinations of items.
- Students will be given a budget form that helps them both reflect on how they can improve from the previous day, and how they can also extract more pepper using the cheapest supplies and labor.
- Give each group a Budget Form.
- Have each group calculate their total cost. (Slides 8-9)
- Give students 10-15 minutes to test their budget plan. (Slide 10)
- After filtering the pepper through the coffee filter, have each group leave their pepper out to dry overnight for another mass check the next day. (Slide 11)
Day 5: Improve and Reflect (45 mins)
- Optional: Review the “Improve” step of the engineering design process. (Slide 3 of the Day 5 Slides)
- Do Now Activity: Give students 5 minutes to weigh their pepper and record this value on their Budget Form. (Slides 4-5)
- Reflection (Slides 6-8):
- Give students 5 minutes to find a group with the same shipment and compare their procedures. (Slide 6)
- Give students 5 minutes to find a group with the opposite shipment and compare their procedures. (Slide 7)
- Have students compare data, reflect on their extraction method, and identify improvements. (Slide 8)
- Give students 5 minutes to mark up their models and make corrections or write their notes. (Slide 9)
- Class Competition Summary (Slide 10)
- Allow all groups to share their final gram amounts and total cost for extraction. (Note: it may be good to have a table/chart/space in the classroom for them to walk up and write this in for all to see).
- Talk with the teams and decide who won the design challenge.
- Optional: Ask the “winning” group to share how they were so successful.
- Provide each student with a Post Test and give them 5 minutes to answer the questions. (Slide 11)
Vocabulary/Definitions
agitation: Stirring or shaking a solution to help dissolve a solute or to mix substances. Example: Agitating a sugar solution by stirring helps the sugar dissolve faster.
aqueous solution: A solution in which water is the solvent. Example: Saltwater is an aqueous solution because water is the solvent.
concentration: The amount of solute dissolved in a given quantity of solvent or solution. Example: A high concentration of salt in water means there is a lot of salt relative to the amount of water.
hydrophilic: Substances that mix well with water; "water-loving." Example: Salt is hydrophilic because it dissolves easily in water.
hydrophobic: Substances that do not mix well with water; "water-fearing." Example: Oil is hydrophobic because it does not dissolve in water.
solute: A substance that is dissolved in a solvent to form a solution. Example: In a saltwater solution, salt is the solute.
solvent: The liquid in which a solute is dissolved to form a solution. Example: In a saltwater solution, water is the solvent.
surface area: The area of the surface of an object. Example: A cube of sugar has a smaller surface area to volume ratio than many small crystals of sugar, meaning the sum of the smaller objects have more area to interact with its surroundings than the single larger object.
Assessment
Pre-Activity Assessment
Pre-Assessment Test: Students will complete a 7-question multiple choice Pre-Test.
Whole-Class Practice Questions (Formative) Assessment
Imagine Ideal Scenarios Worksheet: Students will complete three questions, with their groups, on their Imagine Ideal Scenarios handout to practice their knowledge of aqueous solutions patterns. This can be done first on their own or in their groups, and you can use this as a formative assessment to see how well they applied what they learned in lab to new scenarios. There is an Imagine Ideal Scenarios Answer Key you can refer to.
Pepper Purification Plan Worksheet: During Step 5 of the engineering design process, students must create a step-by-step method to make sugar and salt dissolve in water in their Pepper Purification Plan. This step-by-step method will apply their knowledge of aqueous solutions, and how well they design their method can serve as a formative assessment.
There is a Pepper Purification Plan Shipment “A” Answer Key and Pepper Purification Plan Shipment “B” Answer Key if needed. Methods will vary depending on group budget choices and shipment types, but generally they should follow similar dissolution patterns based on if they needed to dissolve sugar or salt more. The Pepper Extraction Method Rubric can be used both on Day 3 and Day 4 to formatively assess group understandings of dissolving salt and sugar.
If students incorrectly apply their knowledge, (e.g., using cold water to make sugar dissolve instead of hot water) on Day 3, allow them to attempt this in the lab first. Then you may re-teach students so they can revise this method before heading into the lab to test on Day 4.
Post-Activity (Summative) Assessment
Post-Assessment Test: Students will complete an 8-question multiple choice and open-ended Post Test. They will re-assess their knowledge from the pre-test and apply it to a new scenario.
Safety Issues
- Whether doing a demonstration or student-led laboratory experiments, it is important that each student visually sees an adult performing properly at each station. Demonstrate a short portion of each station so visual learners can understand the instructions, and constantly direct them to the printed directions.
- All students should wear safety goggles while in the lab. Although you are not working with harsh chemicals, any splashing could lead to eye damage or burned skin. Be sure to walk through safety procedures such as the eye wash station, what to do with a water burn, and inform the nurse ahead of time in case they are needed.
- If concerned about safety or water temperature heating resources, you can simply have the students do the concentration station, the surface area station, and the agitation station. You can demonstrate the temperature station to the whole class.
Troubleshooting Tips
- If students cannot work well in the lab, you can turn all the stations into demonstrations instead. You can have students record patterns while observing from a distance.
- If there are not enough scales, you can pre-weigh the needed amounts of spices for each station and label them in small cups using a marker. This will also save time if the lab takes longer than expected.
Activity Scaling
- For a more challenging (but also more time-consuming) activity, you can have the groups of four rotate through each station if supplies allow. You can also have them go through more iterations. You could even provide a “mystery shipment” of spices where you know the amounts of each spice, but they must go through trial and error making the solutes dissolve while adhering to the budget.
- If students have not yet been introduced to surface area, and the diagrams on the Day 2 Data sheets are too confusing, you can simply take them out and remove all “surface area” verbiage. You may simply ask students how large or small solutes dissolve in water, and why smaller solutes tend to dissolve faster.
Subscribe
Get the inside scoop on all things Teach Engineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn how to classify materials as mixtures, elements or compounds and identify the properties of each type. The concept of separation of mixtures is also introduced since nearly every element or compound is found naturally in an impure state such as a mixture of two or more substances, and...

In this activity, students investigate the properties of a heterogeneous mixture, trail mix, as if it were a contaminated soil sample near a construction site. This activity shows students that heterogeneous mixtures can be separated by physical means, and that when separated, all the parts will equ...
References
All background knowledge was provided by Sam West, PhD Chemical Engineering candidate in the Kumar and Rosales lab of the University of Texas at Austin Cockrell School of Engineering.
Copyright
© 2025 by Regents of the University of Colorado; original © 2024 University of Texas at AustinContributors
Elizabeth Ademski; Sam West; Veronica RomeroSupporting Program
Research Experience for Teachers (RET), University of Texas at AustinAcknowledgements
This curriculum was developed under National Science Foundation through the Center for Dynamics and Control of Materials: an NSF MRSEC under Cooperative Agreement number DMR-2308817. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Last modified: October 17, 2025






User Comments & Tips